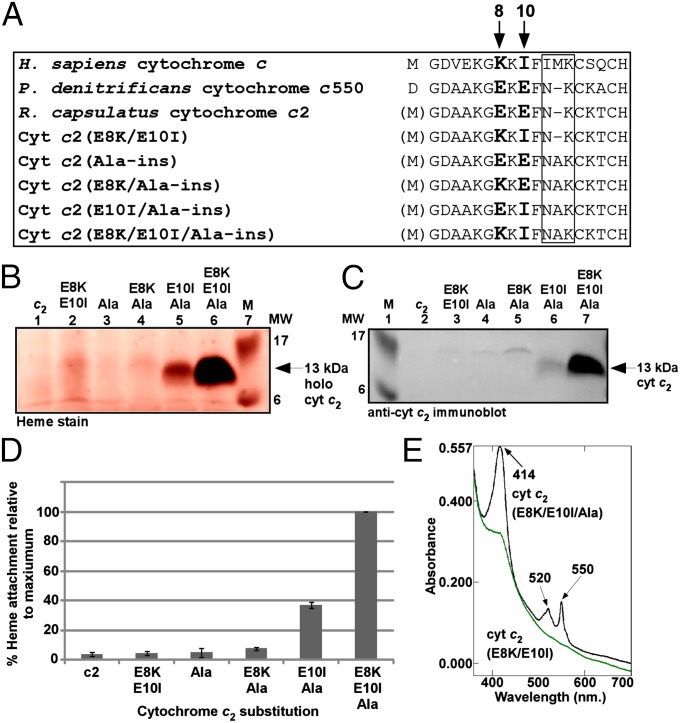
Fig. 4.
Sequence requirements for maturation of a bacterial cytochrome c (cytochrome c2). (A) Amino acid sequence alignment of the N-terminal region (including CXXCH) for the indicated cytochrome c and cytochrome c2 variants. The amino acids mutated in this work are shown in bold and are indicated by arrows. The boxed region corresponds to the location of the alanine insertion; numbering refers to the H. sapiens cytochrome c N-terminal methionine. (B and C) Representative heme staining (B) and anti-cytochrome c2 immunoblot (C) of B-PER extracts showing synthesis of the indicated 12-kDa cytochrome c2 variants; M, molecular weight standards. One hundred micrograms of total protein was loaded in each lane. Prestained molecular weight standards were overlaid in red onto the heme stain. (D) Quantification of the results of heme staining of B-PER extracts from three independent experiments. Percent heme attachment for each indicated variant is relative to synthesis of cytochrome c2(E8K/E10I/Ala-ins), which has been set at 100%. Error bars denote SD. (E) UV-Vis absorption spectra of whole-cell extracts expressing HCCS and the indicated cytochrome c2 variant. Absorption maxima are indicated by arrows.