Key Points
DDR2 regulates the directional migration of neutrophils in 3D collagen matrices, but not on 2D surfaces.
DDR2 regulates directionality through increased metalloproteinase secretion and generation of collagen-derived chemotactic peptide gradients.
Abstract
Neutrophils express a variety of collagen receptors at their surface, yet their functional significance remains unclear. Although integrins are essential for neutrophil adhesion and migration on 2-dimensional (2D) surfaces, neutrophils can compensate for the absence of integrins in 3-dimensional (3D) lattices. In contrast, we demonstrate that the inhibition of the tyrosine-kinase collagen receptor discoidin domain receptor 2 (DDR2) has no impact on human primary neutrophil migration on 2D surfaces but is an important regulator of neutrophil chemotaxis in 3D collagen matrices. In this context, we show that DDR2 activation specifically regulates the directional migration of neutrophils in chemoattractant gradients. We further demonstrate that DDR2 regulates directionality through its ability to increase secretion of metalloproteinases and local generation of collagen-derived chemotactic peptide gradients. Our findings highlight the importance of collagen-derived extracellular signaling during neutrophil chemotaxis in 3D matrices.
Introduction
Neutrophils exhibit the ability to maintain robust migration under a wide array of distinct environmental conditions. For example, by increasing the rate of actin polymerization, neutrophils lacking integrins are able to retain normal migration speeds in 3D environments.1,2 We set out to determine the role of another collagen receptor family, the discoidin domain receptors (DDRs), during neutrophil chemotaxis.
The DDR family is composed of 2 members, DDR1 and DDR2. DDRs are homodimeric receptor tyrosine kinases that bind to triple-helical collagen fibers through a domain similar to discoidin 1 of the social amoeba Dictyostelium discoideum.3,4 On binding to collagen, DDRs become activated and serve as docking sites for many signaling pathways.5 A common outcome after DDR activation is the increase in metalloproteinase (MMP) secretion and collagen rearrangement.6–9 As a consequence, DDR1 and DDR2 have both been associated with metastasis during tumor progression.6 Similarly, DDR1 overexpression has been shown to enhance lymphocyte migration.10,11 However, the mechanism underlying the enhanced leukocyte migration remains unknown.
We show that circulating human primary neutrophils solely express DDR2 and establish that DDR2 regulates the ability of neutrophils to migrate directionally toward chemoattractants in a collagen I matrix. DDR2 activation induces increased MMP secretion, leading to the generation of collagen-derived chemotactic peptides that are poised to form local gradients and enhance neutrophil persistence.
Methods
Isolation of human blood neutrophils
Blood was collected from anonymous healthy donors enrolled in the National Institutes of Health Blood Bank research program, and this study was conducted in accordance with the Declaration of Helsinki. Neutrophils were isolated from heparinized blood by dextran sedimentation and differential centrifugation over Histopaque 1077 (Sigma-Aldrich). Residual erythrocytes were removed by the use of hypotonic lysis. The purity (> 95%) was assessed by May-Grünwald/Giemsa staining.
Inhibitors
DDR activation was inhibited by the use of either anti-DDR2 polyclonal antibodies (ab82856 and ab76967, referenced as Ab1 and Ab2, respectively; Abcam) or by masking the binding sites on collagen with recombinant DDR (rDDR; R&D Systems). The other inhibitors were titrated and used at 350 μg/mL for arginine-threonine-arginine (RTR), a prolyl-glycyl-proline (PGP)–containing peptides inhibitor (Anaspec), 25μM for the pan-MMP inhibitor GM6001 (Millipore), and 100μM for the prolyl endopeptidase (PE) inhibitor Z-PP-CHO (Millipore).
Immunoprecipitation
Neutrophils were lysed in RIPA buffer. A total of 500 μg of protein was mixed with antibodies (DDR2, clone H-108; Santa Cruz Biotechnologies) and magnetic Dynabeads (Invitrogen). The protein-bound beads were washed, subjected to SDS-PAGE, and immunoblotted.
Adhesion assay
We coated 96-well plates with 5 μg/mL collagen I (treated with or without rDDR) in PBS overnight. Neutrophils (2.5 × 105 cells/well) were plated and stimulated for 30 minutes at 37°C. Plates were shaken at 0.5g (200 rpm, radius 1 cm) for 10 seconds on an orbital shaker, and unbound cells were removed. The remaining cells were fixed (4% PFA), stained with crystal violet (5 mg/mL in 2% ethanol; Sigma-Aldrich), and lysed (1% SDS solution). The absorbance at 570 nm was determined using an ELISA plate reader.
EZ-Taxiscan
Rat tail collagen I (BD Biosciences, Bedford, MA) was incubated for 4 hours at 4°C with rDDR or control IgG. Coverslips and chips were coated with collagen for 2 hours. The EZ-Taxiscan chamber (Effector Cell Institute) was used for cell migration analysis as previously described.12
Transwell studies
Transwell chambers (3-μm diameter pore size; Corning) were coated with 1.7 mg/mL collagen or fibrin (Sigma-Aldrich). Neutrophils (2 × 106 cells) were added on top, and the number of cells migrating to either IL-8 (Sigma-Aldrich) or LTB4 (Cayman Chemical) was determined after 6 hours.
3D migration
The 3D chemotaxis assay was adapted from Sixt and Lammermann.13 Neutrophils were embedded in collagen or fibrin as a control. Gels were overlaid with HBSS containing 50nM IL-8 and subsequently imaged on a Zeiss Axiovert S100 microscope. Automated cell tracking was performed as previously described.14 The chemotactic index is defined as the distance moved in the direction of the chemoattractant gradient divided by the total distance moved. The persistence was previously defined.14 We first measured the mean-squared displacement for each cells, a measure of how far a cell migrates in a given time interval. The local slope (α value) provides a measure of persistence, that is, how well the direction of migration is maintained. An α value of 1 means that cells migrate randomly; an α value of 2 means that cells migrate in a straight line.
MMP detection and activity test
The overall MMP activity levels were determined with the EnzCheck Collagenase Assay kit (Invitrogen). To summarize in brief, neutrophils were embedded in collagen and stimulated with 100nM IL-8. The supernatant was collected and added to a solution of 1 mg/mL DQ collagen. The fluorescence generated by collagen hydrolysis was measured at 515 nm. MMP-8 and MMP-9 secretion levels were determined with Quantikine ELISA kits (R&D Systems).
Results
Naive neutrophils express DDR2
Circulating human neutrophils do not express DDR1. DDR1 expression is only detected on priming with GM-CSF for several hours.10 Because neutrophil recruitment occurs within minutes in vivo,15 we reasoned that DDR1 is not relevant for neutrophil recruitment.
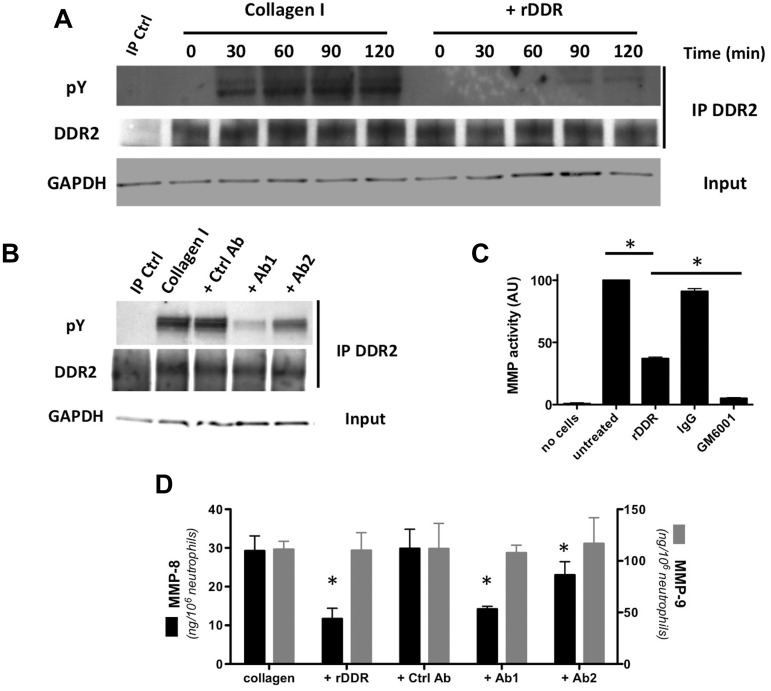
In contrast, DDR2 is readily expressed in freshly isolated human blood neutrophils (Figure 1A). On binding to collagen I, DDR2 is phosphorylated and, consistent with previous studies,16,17 DDR2 phosphorylation is detected 30 minutes after exposure to collagen I and peaks after 90 minutes (Figure 1A; supplemental Figure 1A for quantification [available on the Blood Web site; see the Supplemental Materials link at the top of the online article]). The phosphorylation response is dramatically reduced when DDR binding sites on collagen I are masked with rDDR (Figure 1A). This inhibition occurs in an rDDR dose-dependent fashion (supplemental Figure 1B). Similarly, the treatment of neutrophils with blocking-antibodies that recognize the collagen-binding domain of DDR2 reduces DDR2 phosphorylation levels on collagen engagement (Figure 1B; supplemental Figure 1C for quantification).
Figure 1.
DDR2 is expressed and functional in human primary neutrophils. (A) DDR2 is phosphorylated upon activation by collagen I. Human primary neutrophils were cultured in the presence of collagen I (pretreated with or without rDDR). DDR2 was immunoprecipitated from cell lysates at different times of collagen treatment. Results are representative of 3 independent experiments. (B) DDR2 phosphorylation is inhibited by DDR2 blocking-antibodies. Human primary neutrophils were pretreated for 30 minutes with blocking DDR2 antibodies (50 μg/mL) and cultured in collagen I for 1 hour. DDR2 was immunoprecipitated from cell lysates. Results are representative of 3 independent experiments. (C) MMP secretion is amplified after DDR2 activation. Human primary neutrophils were embedded in collagen I (treated with or without rDDR) and stimulated with 500nM IL-8. The supernatant was collected and added to a solution of DQ collagen. The fluorescence generated by collagen hydrolysis was measured at 515 nm. The pan-MMP inhibitor GM6001 (25μM) was used as control. Results represent the data from 4 independent experiments. *P < .05; Friedman test; Dunn posthoc test. (D) MMP-8, but not MMP-9, secretion is amplified on DDR2 activation. Human primary neutrophils were embedded in collagen and stimulated with 500nM IL-8. DDR2 activity was inhibited either by collagen pretreatment with rDDR or with blocking DDR2 antibodies (pretreatment for 30 minutes). The levels or MMP-8 and MMP-9 in the supernatant were determined by ELISA. Results represent the data from 3 independent experiments. *P < .05; Friedman test; Dunn posthoc test.
DDR2 activation amplifies MMP secretion
DDR2 activation amplifies MMP secretion in many cell types.7,8,18,19 We found that the overall soluble MMP activity also increases after DDR2 activation in neutrophils. In fact, when neutrophils are embedded in collagen, a high soluble collagenase activity in the supernatant is detected after IL-8 stimulation (Figure 1C). In the presence of rDDR, the collagenase activity is significantly reduced (Figure 1C). This activity is also almost completely abolished by the addition of the broad-spectrum MMP inhibitor GM6001 to the medium, confirming that the cleaving factor is soluble MMP (Figure 1C).
Neutrophil granules contain mainly 2 types of MMPs: the collagenase/MMP-8 and the gelatinase-B/MMP-9.20 We therefore determined the levels of secretion of these MMPs by ELISA. Although the secreted MMP-9 levels remain independent of DDR2 activation, MMP-8 secretion levels are significantly reduced after DDR2 inhibition (Figure 1D). Together, these data demonstrate that DDR2 is expressed and active in primary human neutrophils and, upon activation, DDR2 differentially amplifies MMP secretion.
DDR2 is required for neutrophil migration in a 3D collagen lattice
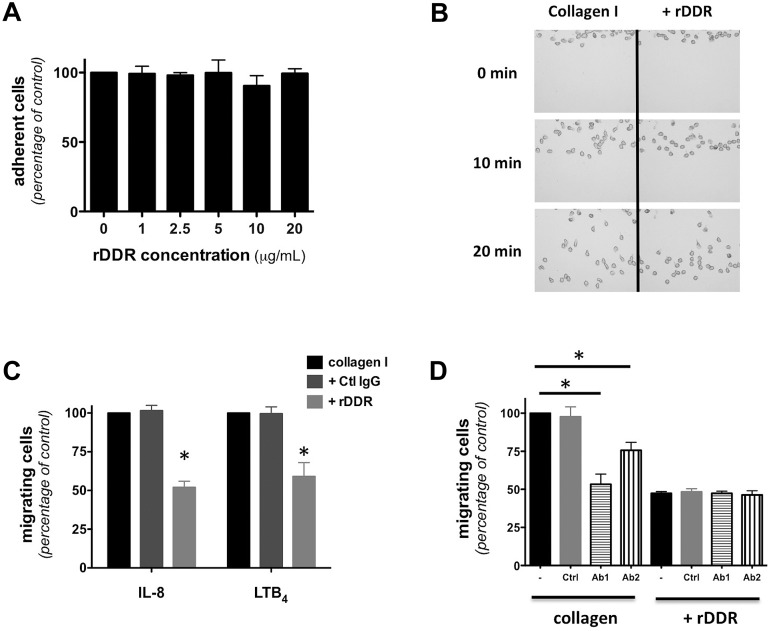
Consistent with previous reports on lymphocytes,21 we found that DDR2 activation is not required for neutrophil adhesion to a 2D collagen I–coated surface (Figure 2A). Similarly, DDR2 inhibition does not alter neutrophil chemotaxis on a 2D collagen-coated surface (Figure 2B; supplemental Figure 2A for quantification; supplemental Video 1). Yet, rDDR treatment significantly reduces neutrophil migration through a collagen I–coated Transwell (Figure 2C). Furthermore, this inhibition occurs in an rDDR dose-dependent fashion and does not occur for neutrophils migrating through a fibrin gel (supplemental Figure 2B), confirming that the inhibition is specific and that rDDR does not have adverse effects on neutrophil migration per se.
Figure 2.
DDR2 regulates neutrophil migration in 3D. (A) Neutrophil adhesion to a collagen-coated surface is not altered by rDDR treatment Human primary neutrophils were plated on collagen I–coated surfaces (treated with or without rDDR) for 30 minutes under IL-8 stimulation. Results represent the relative percentage of adhering cells (average ± SEM) of 3 independent experiments. (B) DDR inhibition does not affect neutrophil migration in 2D. Neutrophil migration to 500nM IL-8 on a collagen I–coated surface (pretreated with or without rDDR) was measured in a EZ-Taxiscan assay. Images were taken every 30 seconds for 30 minutes; 0-, 10-, and 20-minute images are presented. Also see supplemental Video 1. Results are representative of 3 independent experiments. Quantification is presented in supplemental Figure 2A. (C) rDDR treatment inhibits neutrophil migration through collagen-coated Transwells. Migration of human primary neutrophils to 500nM IL-8 or 1μM LTB4 through collagen I–coated Transwells (pretreated of not with rDDR) was determined after 6 hours. Results represent the relative percentage of migrating cells after treatment (average ± SEM) of 4 independent experiments. *P < .05, Friedman test; Dunn posthoc test. (D) Neutrophil treatment with DDR2-blocking antibodies inhibits neutrophil migration through collagen-coated Transwells. Human primary neutrophils were pretreated for 30 minutes with blocking antibodies (or a control antibody). The number of neutrophils migrating to 500nM IL-8 through collagen I–coated Transwells (pretreated with or without rDDR) was determined after 6 hours. Results represent the relative percentage of migrating cells after treatment (average ± SEM) of 4 independent experiments. *P < .05, Friedman test; Dunn posthoc test.
Similarly, treatment of neutrophils with anti-DDR2 blocking antibodies significantly reduces migration through a collagen-coated Transwell (Figure 2D), with a more important inhibition with Ab1, which also shows a more pronounced DDR2 inhibition (Figure 1B). Finally, combining rDDR and anti-DDR2 antibody treatments does not result in cumulative effects (Figure 2D), suggesting that both inhibitors specifically target the same pathway. Together, these findings show that DDR2 is dispensable for migration in 2D environments but important for 3D migration in collagen.
DDR2 regulates neutrophil directionality in 3D
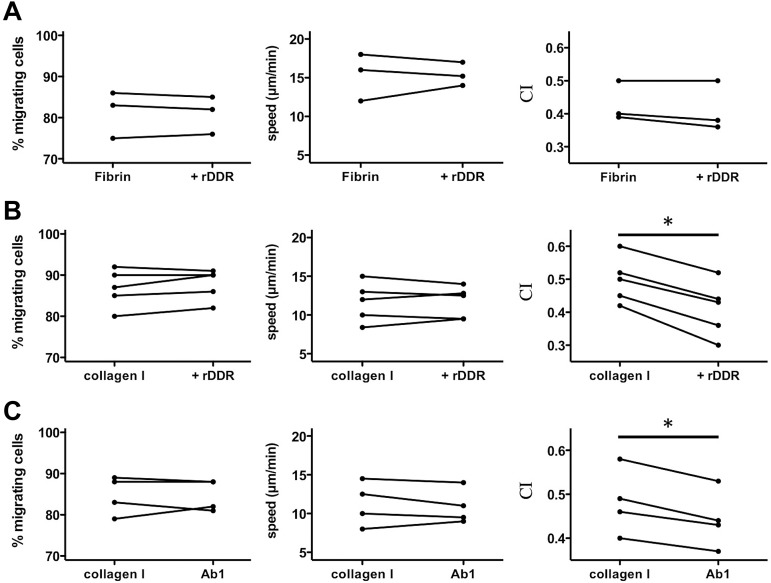
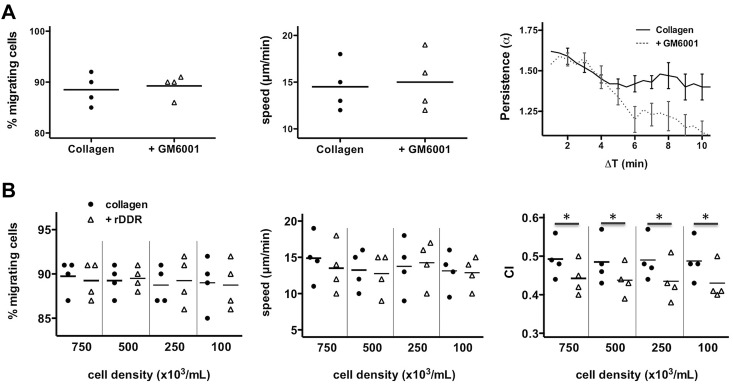
To better understand how DDR2 enhances neutrophil migration, we assessed neutrophil chemotaxis in 3D matrices. As expected, we found that neutrophil chemotaxis in a fibrin gel is not altered after DDR2 inhibition (Figure 3A). Similarly, in a collagen matrix, DDR2 inhibition has no effect on the percentage of migrating neutrophils or on the speed at which they migrate (Figure 3B). Remarkably, however, the chemotaxis index of neutrophils migrating in collagen is significantly reduced after rDDR treatment (Figure 3B; supplemental Videos 2-3). Similar findings are obtained with neutrophils pretreated with a DDR2-blocking-antibody (Figure 3C). We conclude that DDR2 activity regulates directionality during neutrophil chemotaxis in a 3D collagen matrix.
Figure 3.
DDR2 regulates neutrophil directionality in 3D collagen matrices. (A) rDDR treatment does not affect neutrophil chemotaxis in a 3D fibrin gel. Human primary neutrophils were embedded in fibrin (pretreated with or without rDDR). Migration to 50nM IL-8 was recorded (images were taken every 30 seconds with a 5X objective). Migrating cells were tracked for 20 minutes. The percentage, average speed, and CI of migrating neutrophils were determined. Results are representative of 5 independent experiments. (B) rDDR treatment inhibits neutrophil directionality in a 3D collagen matrix. Human primary neutrophils were embedded in collagen I (pretreated with rDDR or not). Migration of neutrophils to 50nM IL-8 was recorded as in panel A. Results are representative of 5 independent experiments. *P = .03, Wilcoxon test. (C) DDR2 inhibition with Ab1 treatment reduces neutrophil directionality in a 3D collagen matrix. Human primary neutrophils were pretreated with blocking DDR2 antibodies or a control IgG for 30 minutes and embedded in collagen I. Migration of neutrophils to 50nM IL-8 was recorded as in panel A. Results are representative of 5 independent experiments. *P = .03, Wilcoxon test.
DDR2-dependent neutrophil chemotaxis is a consequence of DDR2-induced increase in MMP secretion
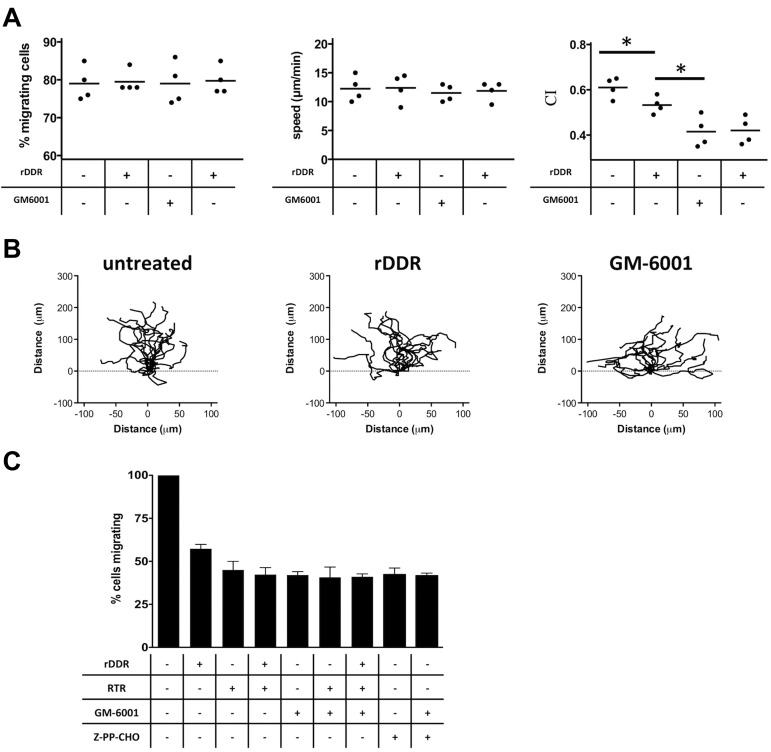
We next set out to determine whether the DDR2-dependent regulation of neutrophil directionality during chemotaxis is MMP-dependent. We first established that the pan-MMP inhibitor GM6001 had no adverse effect on neutrophil migration, as shown when neutrophils migrate through a fibrin gel in Transwell assays (supplemental Figure 3A). Yet, under identical conditions, treatment with GM6001 significantly reduces neutrophil migration through collagen (supplemental Figure 3B). Combining both MMP and DDR inhibitors had no additive inhibitory effects compared with GM6001 treatment alone (supplemental Figure 3B). Finally, when we visualized neutrophil migration in a 3D matrix, we found that, similar to DDR2 activity, MMP activity specifically modulates neutrophil directionality in collagen matrices (Figure 4A-B; supplemental Figure 3C; supplemental Videos 2-4). Together, these findings strongly suggest that DDR2 regulates neutrophil directionality through its ability to increase MMP secretion.
Figure 4.
MMP activity and PGP generation mediate the effects of DDR2 on neutrophil chemotaxis. (A) MMP and DDR2 inhibition affect neutrophil directionality in 3D collagen matrix. Human primary neutrophils were embedded in collagen I (pretreated with or without rDDR). Migration of neutrophils to 50nM IL-8 was recorded as described in Figure 3A. Results are representative of 5 independent experiments. *P < .05, Friedman test; Dunn posthoc test. (B) Neutrophil directionality in a collagen matrix is reduced upon DDR2 or MMP inhibition. The images show paths of individual cells migrating in a 3D collagen I matrix to IL-8 in the presence of the different inhibitors. Data are representative of 5 independent experiments. Also see supplemental Videos 2-4. (C) DDR2- and MMP-dependent migration is dependent on PGP production. Human primary neutrophils were pretreated for 30 minutes with 350 μg/mL RTR, 25μM GM6001, or 100μM Z-PP-CHO. The number of neutrophils migrating to 1μM LTB4 (in the presence of the inhibitors) through collagen I–coated Transwells was determined after 6 hours. Results represent the relative percentage of migrating cells after treatment (average ± SEM) of 4 independent experiments. *P < .05, Friedman test; Dunn posthoc test.
MMP regulates neutrophil chemotaxis via the production of PGP peptides
We next assessed how MMPs modulate neutrophil directionality. First, we envisioned that MMPs could widen the pores of the collagen lattice, facilitating neutrophil migration. However, this hypothesis is unlikely because the collagen density we used was relatively low.22 Moreover, if this indeed occurred, the migration speed should have been affected by treatment with GM6001.23
Alternatively, the product of MMP cleavage could act as a compass for migrating neutrophils. Indeed, MMPs cleave collagen into peptides of 30-100 amino acids in length, which contain the PGP motif. These can be further processed to a minimal PGP (or N-acetyl-PGP) peptide through the action of the PE.24 These PGP peptides have been shown to exhibit chemotactic capacity in vitro and amplify neutrophil recruitment in vivo.25–27 To test this hypothesis, we measured the impact of an inhibitory complementary peptide to PGP-containing peptides, RTR, on neutrophil migration.28,29 We found that RTR treatment specifically inhibits neutrophil migration through collagen (Figure 4C; supplemental Figure 3D). In addition, the levels of inhibition by RTR and GM6001 treatments are comparable, and the combination of both drugs does not lead to cumulative inhibition (Figure 4C). Finally, neutrophil treatment with a PE inhibitor, Z-PP-CHO, significantly reduces neutrophil migration through collagen and the level of inhibition is comparable with the one observed in the presence of GM6001 or RTR (Figure 4C). Such treatments have no impact on neutrophil migration through fibrin (supplemental Figure 3E), demonstrating the absence of adverse effect of the drug on neutrophil migration per se. We conclude that MMP-enhanced chemotaxis is mediated by PGP, and not by other PGP-containing peptides.
Local PGP generation mediates the effects of DDR2 on neutrophil chemotaxis
PGP production could enhance neutrophil chemotaxis in different ways: (1) it could act in an autocrine manner—a local gradient of PGP could be formed and stabilize neutrophil directionality; or (2) it could act in a paracrine fashion—the produced PGP would diffuse and form a secondary gradient, parallel to the primary gradient, thereby enhancing the migration of neutrophils as a population. During chemokinesis (ie, the migration of neutrophils in an isotrope environment), and under the paracrine model, we would expect the produced PGP to diffuse, become uniformly distributed, and therefore have little impact on neutrophil persistence (ie, the capacity of cells to maintain a given direction of motion over time). On the other hand, in the same isotropic environment, under the autocrine model, local PGP gradients would still be formed and inhibition of PGP production would reduce neutrophil persistence. To test this, we analyzed the impact of MMP inhibition on neutrophil persistence (α, defined in “Methods”).14 Neutrophils that migrate in a straight line would have an α value of 2, whereas neutrophils migrating randomly, in a purely diffusive manner, would have an α value of 1. We found that MMP inhibition, which results in the abrogation of PGP production, dramatically reduces the persistence of neutrophils over time (Figure 5A), suggesting that PGP acts in an autocrine fashion.
Figure 5.
DDR2 amplifies neutrophil migration at the single-cell level. (A) MMP regulates neutrophil persistence during chemokenesis in a 3D collagen matrix. Human primary neutrophils were pretreated for 30 minutes with 25μM GM6001 and embedded in collagen I. Migration of neutrophils after a uniform10nM IL-8 stimulation was recorded. The percentage, average speed, and persistence (see “Methods” for details) of migrating neutrophils were determined. Percentage and average speed are from 4 independent experiments. The persistence graph is representative of the 4 experiments. (B) rDDR inhibition is independent of cell density. Human primary neutrophils were embedded at different cell density in collagen I (pretreated with or without rDDR). Migration of neutrophils to 50nM IL-8 was recorded. Results are representative of 5 independent experiments. *P = .03, Wilcoxon test.
To confirm this, we tested the impact of cell density on neutrophil chemotaxis in 3D: decreasing cell densities gradually reduces the effects of the paracrine signals, whereas it does not affect autocrine signals. We found that DDR2-dependent neutrophil chemotaxis is not affected by cell density, confirming that that the DDR2-MMP-PGP pathway acts in an autocrine fashion (Figure 5B).
Discussion
In the present study, we provide novel mechanistic insight into the function of DDR2 during neutrophil chemotaxis in 3D collagen matrices (Figure 6). In response to a gradient of IL-8, neutrophils embedded in collagen release low levels of MMPs and migrate with a low persistence. After DDR2 activation, MMP release, mainly MMP-8, is increased, resulting in substantial cleavage of collagen and generation of PGP-containing peptides, which are further processed into PGP by PE. As a consequence, a local gradient of PGP is produced, purportedly at the leading edge, stabilizing neutrophil persistence and directionality.
Figure 6.
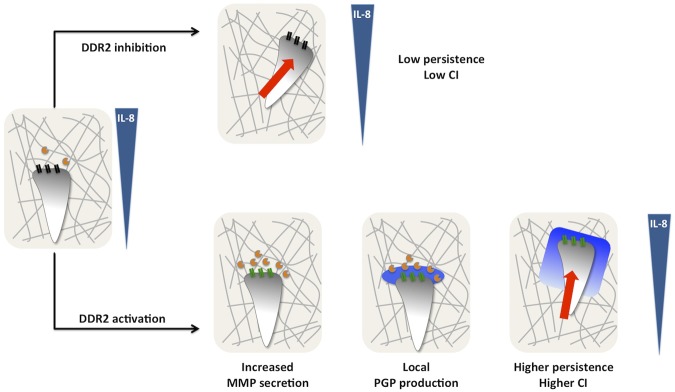
Model depicting DDR2 regulation of neutrophil chemotaxis in a 3D collagen matrix. In response to a gradient of IL-8, neutrophils embedded in collagen (gray lines) release low levels of MMPs (orange circles) and migrate with a low persistence. After DDR2 activation (depicted by the change from black to green), MMP release, mainly MMP-8, is increased resulting in substantial cleavage of collagen and generation of PGP-containing peptides, which are further processed into PGP by PE (blue halo). As a consequence, a local gradient of PGP is produced stabilizing neutrophil persistence and directionality.
The impact of DDR1 on leukocyte migration has previously been suggested in vitro. Indeed, activated lymphocytes show increase migration through collagen.11 Similarly, DDR1 expression in THP-1 cells increases migration in a Transwell device.10 However, DDR1 is not expressed in circulating neutrophils and lymphocytes and is only expressed hours after cell activation.10,30 We speculate that, when neutrophils migrate in vivo toward an inflammation site, DDR1 is not present at the cell surface. By contrast, in physiologic conditions, DDR2 is readily expressed in naive neutrophils. We propose that DDR1 and DDR2 have sequential roles in the inflammation response. Although the role of DDR2 would be primarily to enhance leukocyte migration toward the inflammation site, DDR1 activation would instead occur once leukocytes reach the site of inflammation. There, DDR1 activation would further increase MMP secretion, and PGP production at the inflammation site. PGPs would then form a secondary gradient that enhances recruitment of bystander leukocytes. The DDR1-dependent amplification of the inflammation process would be consistent with the observation that DDR1 overactivation can be associated with inflammatory diseases.31–34
We demonstrate that the effect of DDR2 on neutrophil chemotaxis is related to its ability to increased MMP secretion, namely MMP-8. Interestingly, the putative role of MMPs during leukocyte migration in 3D has been a matter of debate. In some studies authors concluded that MMPs are not involved in 3D leukocyte migration because they have focused primarily on chemokinesis and cell speed.35 On the other hand, studies in which authors used Transwell assay have demonstrated a predominant role of MMP-8 in neutrophil migration.36 Our analyses of the migration properties of neutrophils in the presence and absence of GM6001 provide an explanation for the discrepancy. We clearly establish that MMPs are not required for neutrophil migration per se, as both the speed and the percentage of cells migrating are not altered on GM6001 treatment. Instead, our findings demonstrate that MMPs selectively regulate neutrophil directionality and persistence during chemotaxis and chemokinesis, respectively.
Our work also stresses the importance of local gradients in stabilizing directionality and improving cell migration. We have demonstrated that PGP production enhances neutrophil chemotaxis at a single cell level. This finding suggests that PGP is generated at the leading edge of neutrophils and forms a local gradient that stabilizes directionality, similarly to what has been demonstrated for local ATP gradient37 and suggested for leukotriene B4.38 A local gradient of PGP could be generated in at least 2 ways. First, as MMPs often are released as precursors,39 pro-MMPs could be released homogeneously around cells but cleaved and activated specifically at the leading edge. Second, the local PGP production could be a consequence of the directed secretion of MMPs- and/or PE toward the leading edge.40 Further studies will be required to shed more light into this.
Finally, our findings reveal that different collagen receptors have distinct functions. Integrins are mainly important for adhesion to collagen on 2D surfaces and are dispensable during 3D migration.2 In contrast, we show that inhibition of DDR2 signaling does not alter the ability of neutrophils to adhere to 2D collagen-coated surfaces. Instead, we establish that DDR2 signaling provides directionality signals through MMP secretion and PGP production. Together, these findings provide novel mechanistic insight into how the migration of immune cells is regulated by collagen in an adhesion-independent fashion.
Supplementary Material
Acknowledgments
The authors thank Amy Melpolder and the National Institutes of Health Blood Bank for providing human blood from healthy volunteers. They also thank the members of the Parent laboratory and Dr Phil Murphy for excellent discussions and for valuable input on the manuscript.
This research was supported by the Intramural Research Program of the Center for Cancer Research, NCI, National Institutes of Health.
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.V.A. designed and performed the experiments, analyzed the data, and wrote the manuscript; C.P.M. analyzed the data; S.M.K. performed the experiments; and C.A.P. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carole A. Parent, LCMB/CCR/National Institutes of Health, 37 Convent Dr, Bldg 37, Rm 2066, Bethesda, MD 20892; e-mail: parentc@mail.nih.gov.
References
- 1.Renkawitz J, Schumann K, Weber M, et al. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol. 2009;11(12):1438–1443. doi: 10.1038/ncb1992. [DOI] [PubMed] [Google Scholar]
- 2.Lammermann T, Bader BL, Monkley SJ, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 3.Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;13(Suppl):S77–82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- 4.Abdulhussein R, Koo DH, Vogel WF. Identification of disulfide-linked dimers of the receptor tyrosine kinase DDR1. J Biol Chem. 2008;283(18):12026–12033. doi: 10.1074/jbc.M704592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemeer S, Bluwstein A, Wu Z, et al. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. J Proteomics. 2012;75(12):3465–3477. doi: 10.1016/j.jprot.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18(8):1108–1116. doi: 10.1016/j.cellsig.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Su J, Yu J, Ren T, et al. Discoidin domain receptor 2 is associated with the increased expression of matrix metalloproteinase-13 in synovial fibroblasts of rheumatoid arthritis. Mol Cell Biochem. 2009;330(1-2):141–152. doi: 10.1007/s11010-009-0127-0. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz PA, Jarai G. Discoidin domain receptors regulate the migration of primary human lung fibroblasts through collagen matrices. Fibrogenesis Tissue Repair. 2012;5:3. doi: 10.1186/1755-1536-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin α1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology (Oxford) 2011;50(3):463–472. doi: 10.1093/rheumatology/keq305. [DOI] [PubMed] [Google Scholar]
- 10.Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15(14):2724–2726. doi: 10.1096/fj.01-0359fje. [DOI] [PubMed] [Google Scholar]
- 11.Hachehouche LN, Chetoui N, Aoudjit F. Implication of discoidin domain receptor 1 in T cell migration in three-dimensional collagen. Mol Immunol. 2010;47(9):1866–1869. doi: 10.1016/j.molimm.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19(6):845–857. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sixt M, Lammermann T. In vitro analysis of chemotactic leukocyte migration in 3D environments. Methods Mol Biol. 2011;769:149–165. doi: 10.1007/978-1-61779-207-6_11. [DOI] [PubMed] [Google Scholar]
- 14.McCann CP, Kriebel PW, Parent CA, Losert W. Cell speed, persistence and information transmission during signal relay and collective migration. J Cell Sci. 2010;123(Pt 10):1724–1731. doi: 10.1242/jcs.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng LG, Qin JS, Roediger B, et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol. 2011;131(10):2058–2068. doi: 10.1038/jid.2011.179. [DOI] [PubMed] [Google Scholar]
- 16.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 17.Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283(11):6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Ding T, Zhang J, et al. Expression of discoidin domain receptor 2 (DDR2) extracellular domain in pichia pastoris and functional analysis in synovial fibroblasts and NIT3T3 cells. Mol Cell Biochem. 2006;290(1-2):43–53. doi: 10.1007/s11010-006-9136-4. [DOI] [PubMed] [Google Scholar]
- 19.Olaso E, Lin HC, Wang LH, Friedman SL. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair. 2011;4(1):5. doi: 10.1186/1755-1536-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–3521. [PubMed] [Google Scholar]
- 21.Chetoui N, El Azreq MA, Boisvert M, Bergeron ME, Aoudjit F. Discoidin domain receptor 1 expression in activated T cells is regulated by the ERK MAP kinase signaling pathway. J Cell Biochem. 2011;112(12):3666–3674. doi: 10.1002/jcb.23300. [DOI] [PubMed] [Google Scholar]
- 22.Wolf K, Wu YI, Liu Y, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9(8):893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 23.Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21(12):736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Gaggar A, Jackson PL, Noerager BD, et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180(8):5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weathington NM, van Houwelingen AH, Noerager BD, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Jackson PL, Tanner S, et al. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One. 2011;6(1):e15781. doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin M, Jackson P, Tester AM, et al. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173(1):144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haddox JL, Pfister RR, Sommers CI, Blalock JE, Villain M. Inhibitory effect of a complementary peptide on ulceration in the alkali-injured rabbit cornea. Invest Ophthalmol Vis Sci. 2001;42(12):2769–2775. [PubMed] [Google Scholar]
- 29.Pfister RR, Haddox JL, Blalock JE, Sommers CI, Coplan L, Villain M. Synthetic complementary peptides inhibit a neutrophil chemoattractant found in the alkali-injured cornea. Cornea. 2000;19(3):384–389. doi: 10.1097/00003226-200005000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Dang N, Hu J, Liu X, et al. CD167 acts as a novel costimulatory receptor in T-cell activation. J Immunother. 2009;32(8):773–784. doi: 10.1097/CJI.0b013e3181acea46. [DOI] [PubMed] [Google Scholar]
- 31.Guerrot D, Kerroch M, Placier S, et al. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol. 2011;179(1):83–91. doi: 10.1016/j.ajpath.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerroch M, Guerrot D, Vandermeersch S, et al. Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. FASEB J. 2012;26(10):4079–4091. doi: 10.1096/fj.11-194902. [DOI] [PubMed] [Google Scholar]
- 33.Franco C, Hou G, Ahmad PJ, et al. Discoidin domain receptor 1 (ddr1) deletion decreases atherosclerosis by accelerating matrix accumulation and reducing inflammation in low-density lipoprotein receptor-deficient mice. Circ Res. 2008;102(10):1202–1211. doi: 10.1161/CIRCRESAHA.107.170662. [DOI] [PubMed] [Google Scholar]
- 34.Zeggini E, Reginato AM, Prais A, Thomson W, McLean W, Donn R. Linkage and association studies of discoidin domain receptor 1 (DDR1) single nucleotide polymorphisms (SNPs) in juvenile oligoarthritis. Rheumatology (Oxford) 2004;43(9):1138–1141. doi: 10.1093/rheumatology/keh261. [DOI] [PubMed] [Google Scholar]
- 35.Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102(9):3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 36.Khatwa UA, Kleibrink BE, Shapiro SD, Subramaniam M. MMP-8 promotes polymorphonuclear cell migration through collagen barriers in obliterative bronchiolitis. J Leukoc Biol. 2010;87(1):69–77. doi: 10.1189/jlb.0509361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 38.Afonso PV, Janka-Junttila M, Lee YJ, et al. LTB(4) Is a signal-relay molecule during neutrophil chemotaxis. Dev Cell. 2012;22(5):1079–1091. doi: 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen CA, Hu Z, Barrick B, Shapiro SD. Inducible expression of tissue inhibitor of metalloproteinases-resistant matrix metalloproteinase-9 on the cell surface of neutrophils. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):283–294. doi: 10.1165/rcmb.2003-0034OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.