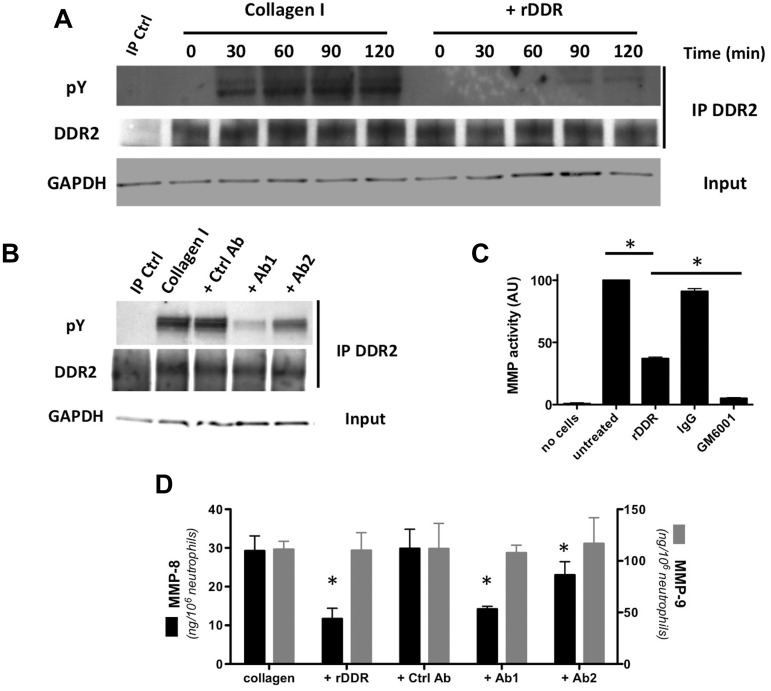
Figure 1.
DDR2 is expressed and functional in human primary neutrophils. (A) DDR2 is phosphorylated upon activation by collagen I. Human primary neutrophils were cultured in the presence of collagen I (pretreated with or without rDDR). DDR2 was immunoprecipitated from cell lysates at different times of collagen treatment. Results are representative of 3 independent experiments. (B) DDR2 phosphorylation is inhibited by DDR2 blocking-antibodies. Human primary neutrophils were pretreated for 30 minutes with blocking DDR2 antibodies (50 μg/mL) and cultured in collagen I for 1 hour. DDR2 was immunoprecipitated from cell lysates. Results are representative of 3 independent experiments. (C) MMP secretion is amplified after DDR2 activation. Human primary neutrophils were embedded in collagen I (treated with or without rDDR) and stimulated with 500nM IL-8. The supernatant was collected and added to a solution of DQ collagen. The fluorescence generated by collagen hydrolysis was measured at 515 nm. The pan-MMP inhibitor GM6001 (25μM) was used as control. Results represent the data from 4 independent experiments. *P < .05; Friedman test; Dunn posthoc test. (D) MMP-8, but not MMP-9, secretion is amplified on DDR2 activation. Human primary neutrophils were embedded in collagen and stimulated with 500nM IL-8. DDR2 activity was inhibited either by collagen pretreatment with rDDR or with blocking DDR2 antibodies (pretreatment for 30 minutes). The levels or MMP-8 and MMP-9 in the supernatant were determined by ELISA. Results represent the data from 3 independent experiments. *P < .05; Friedman test; Dunn posthoc test.