Figure 3.
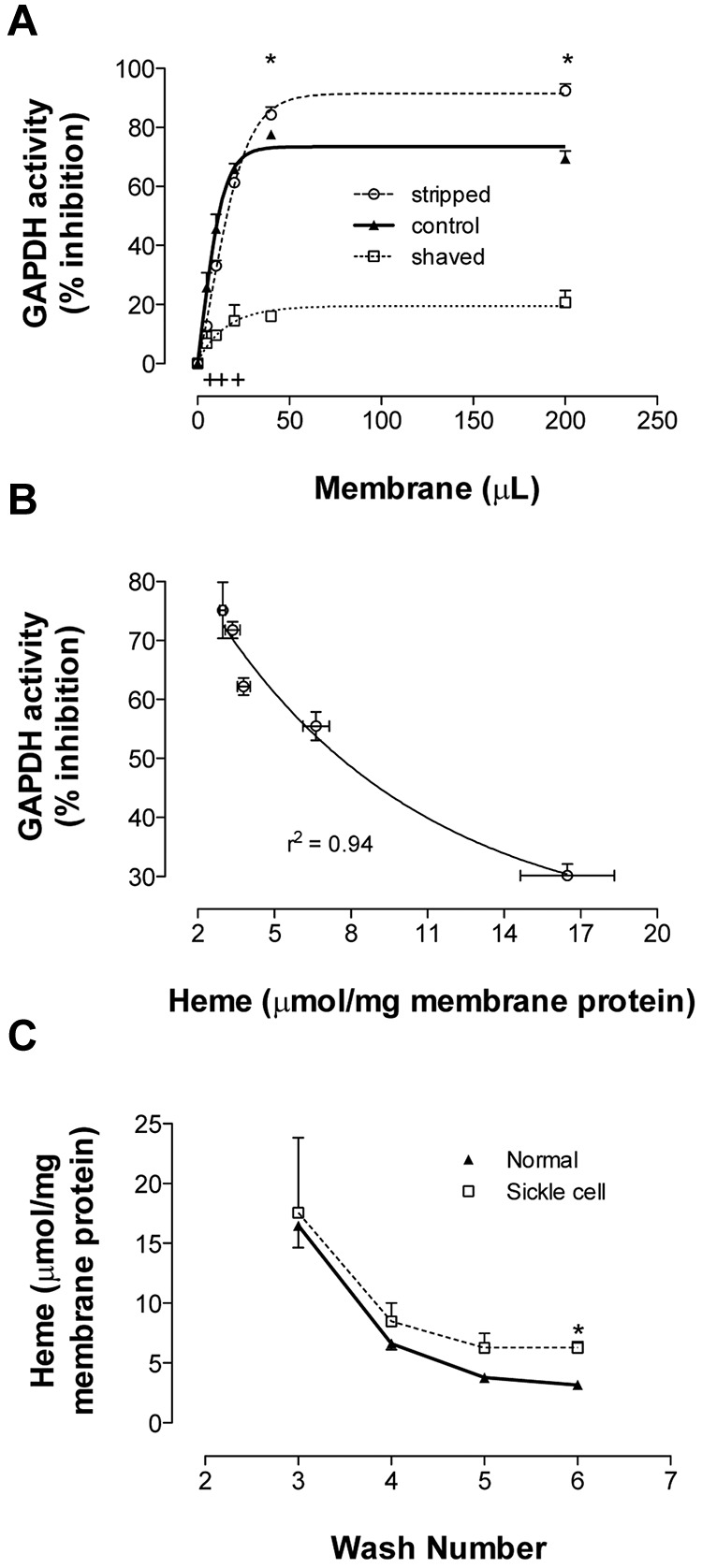
Binding of GAPDH and Hb to RBC membrane. Membranes from fully oxygenated normal RBCs were prepared by hypotonic lysis and alkaline washing. Membranes referred to as “control” were taken from the final (7th) wash. Equal volumes of these membranes were further processed to produce stripped (ie, removal of associated extrinsic membrane proteins) or shaved membranes (ie, digestion of exofacial and cytoplasmic protein domains). (A) To study GAPDH inhibition, the 3 preparations were incubated in enzyme activity buffer, pelleted, and the activity remaining in supernatant was determined (n = 3; mean ± SEM). +P < .05 for control versus shaved; *P < .05 for shaved versus stripped. (B) Samples from each wash (containing progressively less heme) were run in this GAPDH activity assay. The percentage GAPDH inhibition varied inversely with membrane-bound heme (n = 3; R2 = 0.94). (C) Membrane-bound heme was monitored during repeated alkaline washing of membranes purified from fully oxygenated normal or SSRBCs. SS membranes retained heme more than did normal membranes (n = 3-5; mean ± SEM). Note similar globin findings in Figure 4A. *P < .05.
