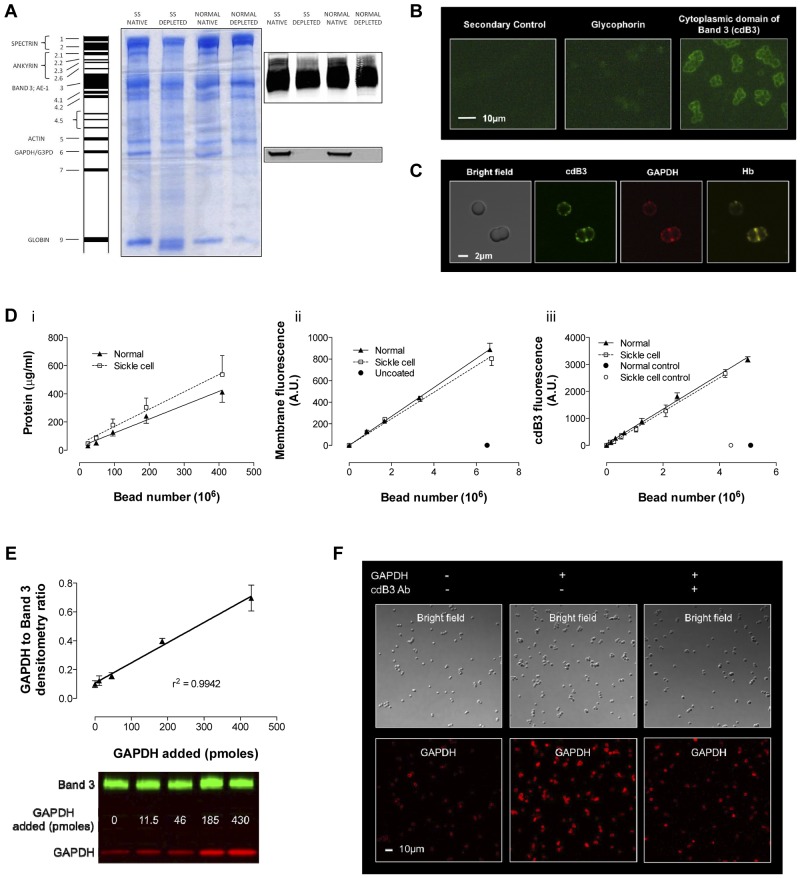
Figure 4.
Model of inverted RBC membrane attached to silica beads. RBC membranes from normal and SSRBCs were prepared by hypotonic lysis and alkaline washing; GAPDH was depleted by further salt washing. (A) Differences were observed in membrane protein banding from normal and SSRBCs. Note that despite salt washing, Hb globin (Band 9) remained associated with SS membranes, which is consistent with membrane-associated heme in Figure 3C. Inverted GAPDH-depleted membranes were subsequently attached to silica beads. (B) Immunofluorescence imaging of glycophorin A (an exofacial label) and cdB3 (a cytoplasmic label) was used to determine membrane orientation (Zeiss Axioskop microscope, 40× objective; AxioCam with AxioVision acquisition software; imaging medium Prolong Gold). (C) Confocal immunofluorescence images of (non–GAPDH-depleted) inverted membrane coated beads demonstrate free access to cdB3, GAPDH, and Hb (FluoViewFV1000 Olympus confocal microscope, PlanApo 60×/1.40 oil objective, 2.4× zoom; Flouview acquisition software; imaging medium Prolong Gold). (D) The model was further characterized by comparing membrane protein, membrane volume, and B3 number (as a function of bead count) between preparations from normal and SSRBCs. Although the membrane protein load per bead was greater for SS preparations, the membrane volume and B3 count was invariant. (E) To evaluate GAPDH binding to the bead model, we determined the ratio between membrane B3 and membrane-bound GAPDH as a function of [GAPDH] in solution; as expected, this relationship is linear. (F) GAPDH binding specificity for B3 was qualitatively evaluated by confocal immunofluorescence, which illustrates variation in GAPDH binding ± preincubation with antibody to the cdB3 NH2 terminus (GAPDH-binding site; FluoViewFV1000 Olympus confocal microscope, PlanApo 60×/1.40 oil objective; Flouview acquisition software; imaging medium Prolong Gold).