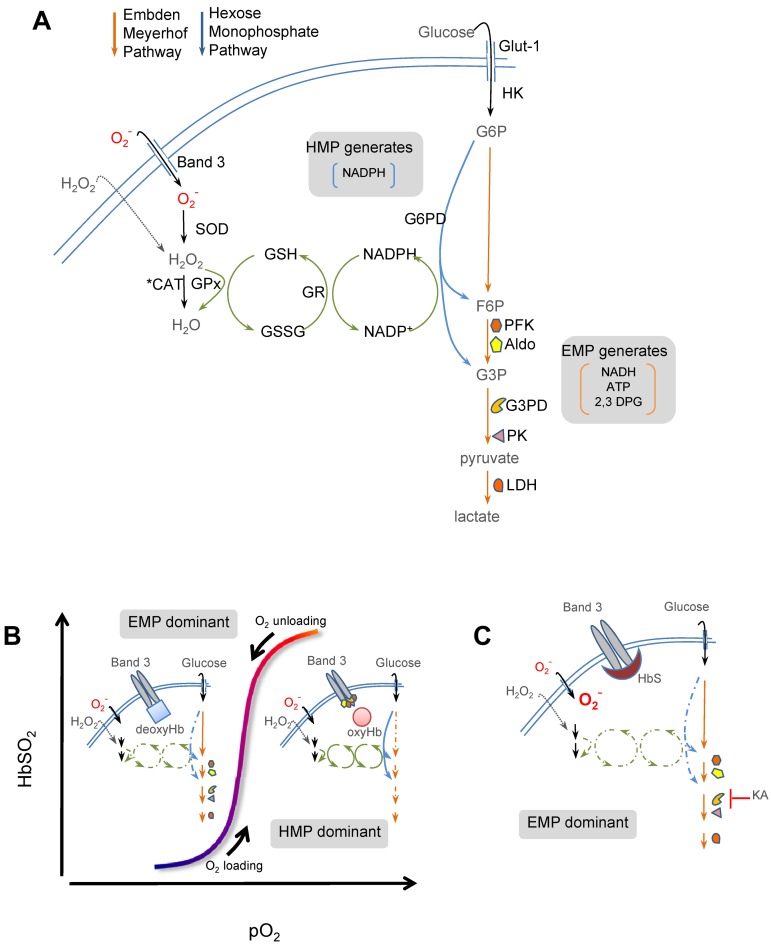
Figure 7.
Simplified scheme of cdB3-based control of glucose metabolism in RBCs. (A) Energy metabolism in RBCs proceeds through either the EMP pathway (orange arrows) or the HMP pathway (blue arrows; also known as the pentose shunt). Both share G6P as initial the substrate. The HMP is the sole source of NADPH in RBCs and generates fructose-6-phosphate (F6P) or glyceraldehyde-3-phosphate (G3P), which rejoin the EMP before glyceraldehyde-3-phosphate dehydrogenase (G3PD/GAPDH), a key regulatory point. The EMP generates NADH (used by met-Hb reductase) and ATP (to drive ion pumps) and 2,3-DPG (to modulate Hb p50). Hydrogen peroxide (H2O2) and O2− are the principal endogenous reactive oxygen species (ROS) encountered by RBCs. Both ROS may be generated internally (not shown); however, only H2O2 can cross the membrane directly. O2− enters RBCs through Band 3 (anion exchange protein 1 or AE-1). H2O2 and O2− are ultimately reduced to water by catalase or GSH peroxidase (GPx). (B) O2 content modulates EMP and HMP balance via reciprocal binding for cdB3 between deoxy-Hb and key EMP enzymes (PFK, Aldo, GAPDH, PK, and LDH).16,17 In oxygenated RBCs (right half of stylized oxygen dissociation plot), sequestration to cdB3 inactivates these EMP enzymes, resulting in HMP dominance and maximal NADPH (and thus GSH) recycling capacity. In deoxygenated RBCs (left half of oxygen dissociation plot), deoxy-Hb binding to cdB3 disperses these EMP enzymes, creating G6P substrate competition and thereby constraining HMP flux, limiting NADPH and GSH recycling capacity, and weakening resilience to ROS such as O2−. (C) In SSRBCs, SSHb binds abnormally avidly to the RBC membrane at cdB3.6,7 We hypothesized that this biases normal EMP/HMP cycling (disfavoring HMP), rendering SSRBCs vulnerable to oxidant attack. In support of this, we found that by blocking the EMP with KA (at the point normally inhibited by cdB3-GAPDH binding) restored resilience to oxidative loads, presumably by lifting the G6PD substrate constraint.