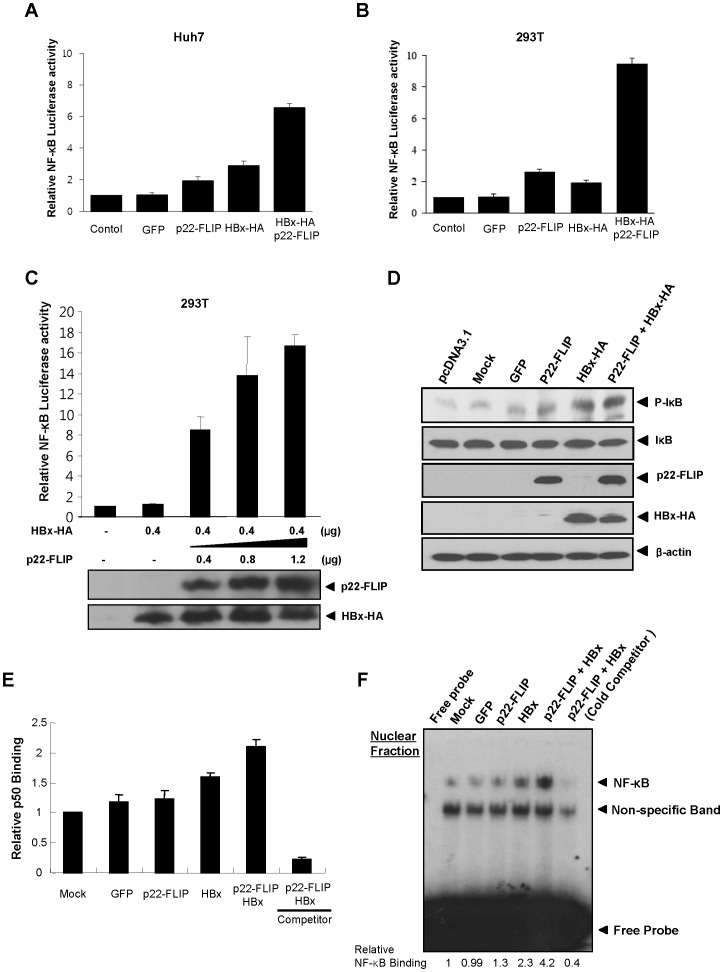
Figure 2. p22-FLIP synergistically up-regulates HBx-mediated NF-κB signaling.
(A–B) Relative NF-κB activity after co-transfection of pNF-κB-Luc and p22-FLIP plasmid with/without HBx-HA plasmid in Huh7 and 293T cells, respectively. pEGFP were transfected for the monitoring of transfection efficiency and negative control. (C) Dose-dependent activation of HBx-mediated NF-κB by p22-FLIP. pNF-κB-Luc (0.25 µg) and HBx-HA plasmid (0.4 µg) were co-transfected with increasing amounts of p22-FLIP (0∼1.2 µg) in 293T cells. Total transfected DNA amounts were adjusted using the empty vector (pCMV). The expression levels of p22-FLIP and HBx were determined by Western blot. (D) The level of phospho-IκB (P-IκB) was determined by western blot. The plasmids of p22-FLIP (1 µg) and HBx-HA (1 µg) were co-transfected in Huh7 cells. After 48 hours, the levels of P-IκB and total IκB were analyzed by western blot. (E) NF-κB ELISA was measured by p50 ELISA using nuclear extracts. The interaction of plate-bound NF-κB consensus DNA oligomer and NF-κB subunit (p50) in nuclear extracts was measured by chemiluminescence. (F) NF-κB electrophoretic mobility shift assay (EMSA). The [P32]-labeled NF-κB consensus DNA oligomer probe was reacted with nuclear extracts (3 µg) in vitro. Non-labeled NF-κB consensus oligomer (30 fold) was used for cold competition. Relative binding affinity was calculated by densitometry.