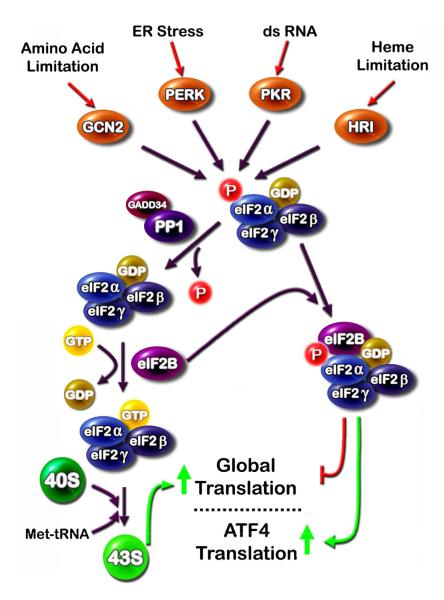
Figure 1.
Several cellular stress signals are transduced through four eukaryotic initiation factor 2α (eIF2α) kinases. (i) The eIF2α kinases general non-derepressible 2 (GCN2), double-stranded RNA-activated protein kinase (PKR), double-stranded RNA activated protein kinase-like ER kinase (PERK), and heme-regulated inhibitor kinase (HRI) are activated by a wide spectrum of cell stress signals and all phosphorylate eIF2α on serine 51. (ii) Phospho-eIF2α binds eIF2B in a non-functional complex and leads to (iii) suppression of global translation, but a paradoxical increase in translation of specific mRNA species, such as that for activating transcription factor 4 (ATF4). De-phosphorylation of eIF2α is mediated by protein phosphatase 1 (PP1) which is targeted to eIF2α by growth arrest and DNA damage-inducible 34 (GADD34).