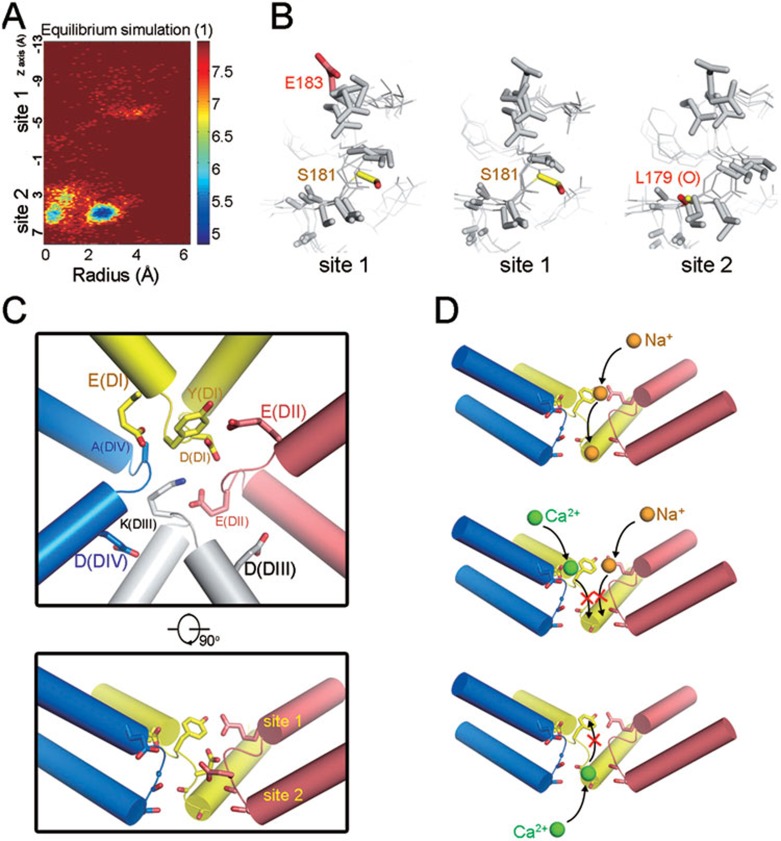
Figure 4.
Na+ passes through the vestibule of NavRh SF in an asymmetric manner. (A) The 2D free energy estimated from the negative logarithm of radial-axial densities of Na+ in the whole SF. (B) Superposition of the four protomers of the pore domain in the representative simulated structures when a Na+ binds to site 1 (left and middle panels) or site 2 (right panel). The groups involved in Na+ binding are highlighted in colored sticks. (C) Schematic representation of modeled mammalian Nav channel structure in two perpendicular views. The outer negatively charged ring and Tyr constitute site 1. The conserved residues DEKA from the four domains form the inner ring beneath site 1. Site 2 consists of eight carbonyl oxygen atoms. (D) The simulation-derived mechanisms by which Nav channels discriminate Na+ and Ca2+ as well as the block of Na+ permeation by Ca2+. An Na+ ion enters SF and binds to site 1 and site 2 sequentially; a Ca2+ ion can be well coordinated at site 1 when entering from extracellular side, or at site 2 when coming from intracellular side. The transfer of Ca2+ between the two sites is suppressed by a high-energy barrier.