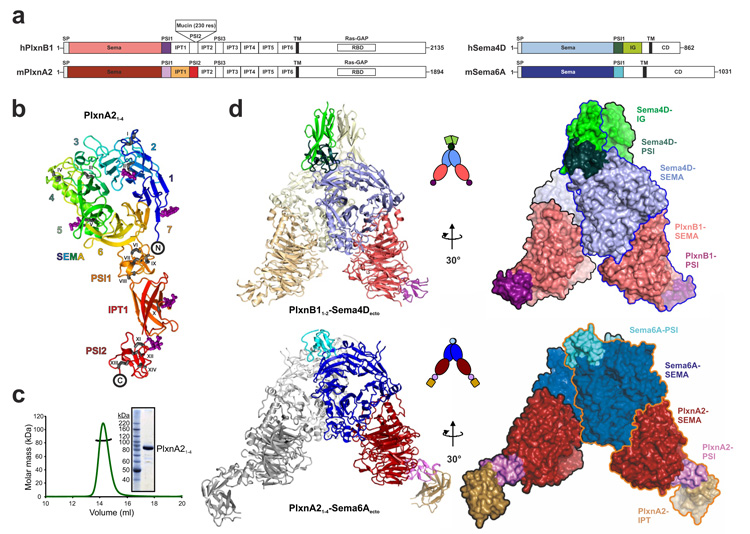
Figure 1. The semaphorin–plexin complexes share a common architecture.
a, Schematic domain organization of human PLXNB1, mouse PlxnA2, human SEMA4D and mouse Sema6A. PLXNB1 contains an additional mucin-like domain inserted into the PSI2 domain. SP, signal peptide; TM, transmembrane. The domains included in the crystallization constructs are coloured. b, Ribbon representation of PlxnA21–4 ‘rainbow’ colour ramped from blue (N terminus) to red (C terminus) with the β-propeller blades numbered. N-linked glycans are shown in magenta ball-and-stick representation and the 14 disulphide bridges (black stick presentation) are marked with Roman numbering. c, Multi-angle light scattering indicates an experimental molecular mass (black line) of 83.7 ± 0.8 kDa for PlxnA21–4 (green line; elution profile, axis not shown) as observed by SDS–PAGE (inset) and in agreement with the theoretical molecular mass for a monomer (85 kDa). d, Ribbon representation (left panel), cartoon drawing (middle panel) and surface representations with individual protein chains indicated by an outline (right panel) of the PLXNB11–2–SEMA4Decto and PlxnA21–4–Sema6Aecto complexes. Domains are coloured as in Fig. 1a.