Figure 1. Lipids and their metabolizing enzymes in the regulation of mammalian autophagy.
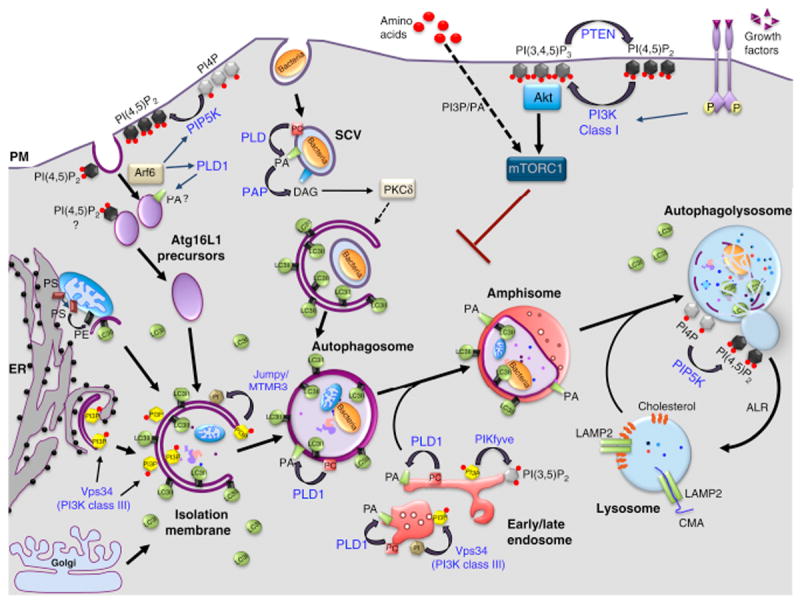
Macroautophagy begins by the formation of an isolation membrane (IM), which sequesters cytoplasmic substrates and seals to form autophagosomes (APs). APs undergo a maturation process through fusion with early and late endosomes, forming amphisomes. These hybrid organelles eventually fuse with lysosomes (autophagolysosomes, AL), where the degradation of sequestered material occurs. At the end of this process, ALs give rise to lysosomes through a recycling process called autophagic lysosome reformation (ARL). Several sources of membrane have been proposed for the IM: endoplasmic reticulum (ER), plasma membrane (PM), mitochondria (M) and Golgi apparatus (GA). A popular model posits that APs are nucleated at specialized sites of the ER referred to as “omegasomes” (Om), which are generated through a Vps34/PI3P-dependent mechanism and disengage to form the IM. Plasma membrane-derived Atg16L-positive vesicles can also contribute to the formation of IMs after their homotypic fusion. The small GTPase Arf6 modulates this process by activating two lipid enzymes, PLD1 and PIP5K, which produce PA and PI(4,5)P2, respectively. LC3 family members are lapidated through a covalent bond to phosphatidylethanolamine (PE) on the IM and APs. The pool of LC3-like molecules located on the outer membrane is released before APs fuse with the lysosomes, while the one located in the inner membrane is degraded alongside the AL cargoes. Other lipids (black) and their corresponding metabolizing enzymes (blue) are shown in the figure, based on their implication in starvation-induce autophagy or non-canonical forms of autophagy, such as that induced by Salmonella. Lysosomes are also essential for chaperone-mediated autophagy (CMA) and microautophagy (not shown on the figure). In CMA, proteins harboring the peptide motif KFERQ are selectively recognized by a cytosolic chaperone, Hsc70, targeting these proteins to the lysosomal membrane, where they can be internalized for degradation through the formation of a complex with LAMP2. In contrast, microautophagy involves the direct engulfment of cytoplasmic material, including proteins, into lysosomes through mechanisms involving the formation of intraluminal vesicles. SCV, Salmonella containing vacuole.
