Abstract
Glutathione is an important antioxidant and has many important functions in plant development, growth and defense. Glutathione synthesis and degradation is highly compartment-specific and relies on the subcellular availability of its precursors, cysteine, glutamate, glycine and γ-glutamylcysteine especially in plastids and the cytosol which are considered as the main centers for glutathione synthesis. The availability of glutathione precursors within these cell compartments is therefore of great importance for successful plant development and defense. The aim of this study was to investigate the compartment-specific importance of glutathione precursors in Arabidopsis thaliana. The subcellular distribution was compared between wild type plants (Col-0), plants with impaired glutathione synthesis (glutathione deficient pad2-1 mutant, wild type plants treated with buthionine sulfoximine), and one complemented line (OE3) with restored glutathione synthesis. Immunocytohistochemistry revealed that the inhibition of glutathione synthesis induced the accumulation of the glutathione precursors cysteine, glutamate and glycine in most cell compartments including plastids and the cytosol. A strong decrease could be observed in γ-glutamylcysteine (γ-EC) contents in these cell compartments. These experiments demonstrated that the inhibition of γ-glutamylcysteine synthetase (GSH1) – the first enzyme of glutathione synthesis – causes a reduction of γ-EC levels and an accumulation of all other glutathione precursors within the cells.
Keywords: Arabidopsis, cysteine, glutamate, glutathione, glycine
Introduction
The tripeptide glutathione (γ-glutamylcysteinylglycine) is an important antioxidant in plants and is of great importance for plant metabolism and plant defense. It plays important roles in the detoxification of reactive oxygen species (Noctor and Foyer 1998; Tausz et al. 2004; Foyer and Noctor 2009; Szalai et al. 2009; Noctor et al. 2011), xenobiotics, herbicides (Edwards et al. 2005; DeRidder and Goldsbrough 2006), heavy metals such as cadmium (Zawoznik et al. 2007; Ammar et al. 2008; DalCorso et al. 2008; Dučić et al. 2008; Nocito et al. 2008), and protects proteins from oxidation through a process called glutathionylation (Hurd et al. 2005a, 2005b). Glutathione is also involved in stress signaling and defense gene expression (Foyer et al. 2001; Maughan and Foyer 2006; Foyer and Noctor 2009; Szalai et al. 2009; Noctor et al. 2011). Considering the importance of glutathione, its availability, synthesis, and degradation in plants is of great importance for successful plant development and defense.
Glutathione synthesis underlies, like its degradation, highly compartment-specific pathways (Cairns et al. 2006; Pasternak et al. 2008; Noctor et al. 2011). Glutathione synthesis in plants takes place in two adenosine triphosphate (ATP)-dependent steps. In the first step, cysteine is linked together with glutamate by γ-glutamylcysteine synthetase (GSH1; also referred to as γ-ECS in some literature) to form γ-glutamylcysteine (γ-EC). As GSH1 is encoded by a single gene, which is exclusively targeted to plastids in Arabidopsis, it is speculated that this reaction takes place exclusively in plastids in Arabidopsis plants (Wachter et al. 2005). The situation is less clear in other plant species as GSH1 has also been detected in leaf extracts (e.g. wheat) after chloroplast isolation (Noctor et al. 2002) and as it is encoded by more than one gene in some plant species (e.g. Oryza sativa, Populus trichocarpa). Thus, it appears that in other plant species GSH1 might also be active in cell compartments (e.g. cytosol) other than the chloroplast (Hell and Bergmann 1990; Noctor et al. 1998; Noctor et al. 2002; Kopriva 2006). In the second, step glycine is linked to γ-EC by glutathione synthetase (GSH2; also referred to as GSHS in some literature) to form the final product, glutathione. As GSH2 is targeted to plastids and the cytosol in Arabidopsis (Wachter et al. 2005) it seems that this step takes place, to different extents, in both plastids and the cytosol (Noctor et al. 2002; Sugiyama et al. 2004). Thus, considering the current knowledge about glutathione synthesis, plastids and the cytosol can be considered the main centers of glutathione synthesis in plants (Noctor et al. 2011). Cysteine and subsequently γ-EC are the limiting precursors for glutathione synthesis as it has been demonstrated that both the artificial elevation of cysteine (Gullner et al. 1999; Harms et al. 2000; Bloem et al. 2004; Bloem et al. 2007; Zechmann et al. 2007a, 2008a) and the overexpression of genes and enzymes involved in cysteine synthesis (Harms et al. 2000; Noji and Saito 2002; Wirtz and Hell 2007) increased glutathione in plants. Nevertheless, it has been shown, under certain conditions (absence of photorespiration, darkness), that glycine can also limit glutathione synthesis (Noctor et al. 1997a, 1997b). Further, since the first step of glutathione synthesis in Arabidopsis seems to take place exclusively in plastids, the availability of cysteine and glutamate in plastids is essential for the synthesis of γ-EC and subsequently glutathione. In addition, as the second step of glutathione synthesis takes place in plastids and the cytosol, the availability of glycine and γ-EC determines glutathione synthesis in these two cell compartments. Thus, it becomes obvious that the subcellular determination of glutathione precursors and the correlation to the compartment-specific glutathione status is essential for the better understanding of glutathione synthesis and its role in plant development and defense. Despite our deep understanding of the compartmentation of glutathione synthesis, the compartmentation of glutathione degradation is still strongly debated. Most attention continues to be paid to apoplastic and vacuolar routes, but another possibility of glutathione degradation also involves the cytosol (Noctor et al. 2011). One possible pathway of glutathione degradation is catalysed by γ-glutamyl transpeptidase (also referred to as γ-glutamyl transferase in some literature), (GGT) which transfers glutamate from glutathione to other dipeptides. Isoforms of GGT occur at the plasmalemma, within vacuoles and the apoplast (Foyer et al. 2001; Noctor et al. 2001; Storozhenko et al. 2002; Shaw et al. 2005; Ohkama-Ohtsu et al. 2007a, 2007b; Ferretti et al. 2009; Destro et al. 2011). Another pathway of glutathione degradation is facilitated by a carboxypeptidase that has been detected within vacuoles of barley leaves (Wolf et al. 1996) and removed glycine from glutathione. The remaining dipeptides in both pathways could then be metabolized by a dipeptidase to the component amino acids (Foyer et al. 2001). Other pathways of glutathione degradation could be facilitated by γ-glutamyl cyclotransferase and 5-oxoproline, which produce free glutamate (Martin and Slovin 2000; Ohkamu-Ohtsu et al. 2008), or by phytochelatin synthase, which could be especially important for the breakdown of glutathione in situations where conjugated glutathione accumulates within the cytoplasm (Grzam et al. 2006; Blum et al. 2007, 2010). Both reactions seem to take place in the cytosol and would be alternative pathways to the degradation of glutathione in the vacuole and the apoplast (Noctor et al. 2011).
The aim of this study was to investigate the subcellular distribution of glutathione precursors during situations of impaired glutathione synthesis in order to obtain more information about the compartment-specific importance of glutathione precursors for glutathione synthesis and degradation. The hypothesis that low levels of glutathione induced by the inhibition of GSH1 are caused by low levels of γ-EC rather than by reduced production of the other glutathione precursors was tested. Therefore the subcellular distribution of cysteine, glutamate, glycine and γ-EC was studied and correlated with the compartment-specific distribution of glutathione in the Arabidopsis mutant pad2-1 and one complemented line (OE3, which is equivalent to line 3 described in Parisy et al. 2007) where glutathione synthesis was restored by genetic complementation of pad2-1 with wild-type GSH1 cDNA (Parisy et al. 2007; Zechmann et al. 2008b). The pad2-1 mutant shows impaired glutathione metabolism due to a single point mutation of the gene encoding GSH1 thus leading to about 80% less glutathione contents when compared with wild type (Parisy et al. 2007; Zechmann et al. 2008b). The situation in the pad-2-1 mutant was compared with plants where glutathione synthesis was artificially inhibited by buthionine sulfoximine (BSO) for 2 days in order to investigate the effects of long and short term inhibition of glutathione synthesis on glutathione precursor contents. BSO inhibits GSH1, thus leading to a strong decrease in glutathione contents in plants (Müller et al. 1999; Gullner and Dodge 2000; Meyer and Fricker 2002; Hartmann et al. 2004; Senda and Ogawa 2004; Zechmann et al. 2006b). During BSO-treatment the investigations of the subcellular distribution of glutathione precursors in the above described mutants gave more detailed insight into the compartment-specific importance of glutathione precursors during situations of impaired glutathione production and for glutathione synthesis and degradation in general.
Results
Cysteine
In wild type plants (accession Col-0) cysteine contents were found to be highest in mitochondria, peroxisomes and the cytosol. Significantly lower levels in this accession were found in plastids, nuclei and vacuoles (Table 1), whereas no cysteine was detected in cell walls and intercellular spaces (Figure 1). Significantly increased amounts of cysteine were found in most cell compartments of the pad2-1 mutant, whereas similar levels of cysteine were found in all cell compartments of the complemented line OE3 when compared with wild type. The strongest increase of cysteine in the pad2-1 mutant when compared with the wild type was found in nuclei (127%), followed by mitochondria and the cytosol (both 90%), plastids (86%) and peroxisomes (60%). Unchanged levels were found in vacuoles (Figures 1, 2). The treatment of wild type plants with BSO induced a strong increase in cysteine contents in peroxisomes (294%), plastids (236%), cytosol (222%), mitochondria (207%), nuclei (141%) and vacuoles when compared with control wild type plants (133%; Figure 2).
Table 1.
Quantitative analysis of glutathione precursor specific labeling in Arabidopsis wild type plants
| Cysteine | Glutamate | γ-EC | Glycine | |
|---|---|---|---|---|
| Mitochondria | 8.3 ± 0.8a | 37.4 ± 6c | 70.4 ± 12a | 12.8 ± 1b |
| Plastids | 4.3 ± 0.3b | 67 ± 2b | 63 ± 4a | 8.9 ± 0.3cd |
| Nuclei | 4.5 ± 0.4b | 83.7 ± 3a | 71 ± 8a | 10.3 ± 1bc |
| Peroxisomes | 6.3 ±0.3a | 65.2 ± 5b | 61 ± 7a | 12.2 ± 1b |
| Cytosol | 7.2 ± 0.6a | 72.6 ± 4ab | 70 ± 7a | 17 ± 1a |
| Vacuoles | 3.4 ± 0.2b | n.d. | 18 ± 0.7b | 7.1 ± 0.4d |
Data are means with standard errors and document changes in the density of gold particles per μm2 bound to cysteine, glutamate, γ-EC and glycine in the respective Arabidopsis leaf cells. Significant differences between the samples are indicated by different lowercase letters; samples that are significantly different from each other have no letter in common. P < 0.05 was regarded significant analyzed by the Kruskal-Wallis test, followed by post hoc comparison according to Conover. n > 20 for peroxisomes and vacuoles and n > 60 for all other cell structures. n.d., not detected.
Figure 1.
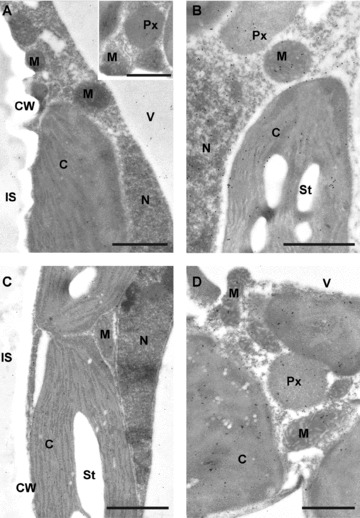
Transmission electron micrographs of mesophyll cells from Arabidopsis leaves after immunogold labeling of cysteine. Different amounts of gold particles can be observed between the Arabidopsis wild-type Col-0 (A), the pad 2-1 mutant (B), the complemented line OE3 (C), and Col-0 after the treatment with 2mM buthionine sulfoximine (BSO) for 48 h (D). Note that cells of the OE3 line (C) show similar amounts of gold particles bound to cysteine in all organelles, whereas the pad 2-1 mutant (C) and wild type plants treated with BSO (D) contain higher amounts of gold particles bound to cysteine in all cell compartments when compared to Col-0 (A). C, chloroplasts; CW, cell walls; IS, intercellular spaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles. Sections were post stained with uranyl acetate for 15 s. Bars: 1 μm.
Figure 2.
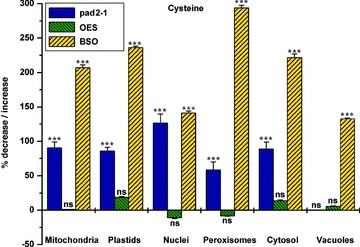
Quantitative analysis of cysteine specific labeling in the glutathione deficient mutant pad2-1, the complemented line OE3 and after the treatment of wild type plants with buthionine sulfoximine (BSO). Graph shows means with standard errors and documents changes in the density of gold particles bound to cysteine in the respective Arabidopsis leaf cells when compared to control wildtype plants. Significant differences were calculated using the Mann-Whitney U-test; (***) indicates significance at the 0.001 level of confidence. P > 0.05 was considered as not significant (ns); n > 20 for peroxisomes and vacuoles and n > 60 for all other cell structures.
Glutamate
In wild type plants (accession Col-0) glutamate-specific labeling was greatest in the nuclei and cytosol and lowest in mitochondria. Intermediate labeling in this accession was found in plastids and peroxisomes (Table 1), whereas no glutamate was detected in vacuoles, cell walls, or intercellular spaces (Figure 3). Glutamate could also be detected along the membranes of the endoplasmic reticulum (inset in Figure 3A). Glutamate-specific labeling significantly increased in most cell compartments of the pad2-1 mutant when compared to wild type plants. In the pad2-1 mutant, the strongest increase in glutamate contents was observed in mitochondria (165%), followed by peroxisomes (66%), the cytosol (31%), and nuclei (22%). No significant change in glutamate specific labeling was observed in plastids in the pad2-1 mutant when compared to wild type. In the complemented line, OE3 glutamate was significantly increased in mitochondria (112%) and plastids (21%) when compared to wild type. No significant change was found in nuclei, peroxisomes or the cytosol in the complemented line when compared to the wild type (Figures 3,4). BSO-treatment of wild type plants caused a significant increase in glutamate contents in comparison to control wild type plants in mitochondria (84%) and the cytosol (52%) whereas glutamate contents in all other cell compartments remained unaffected (Figure 4).
Figure 3.
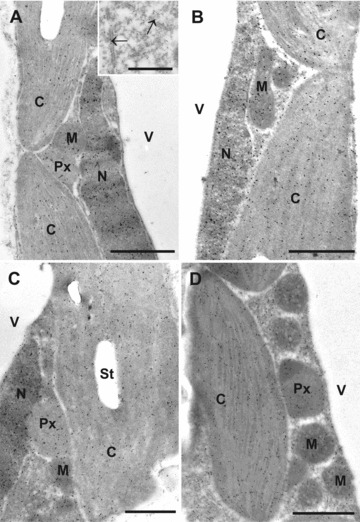
Transmission electron micrographs of mesophyll cells from Arabidopsis leaves after immunogold labeling of glutamate. Different amounts of gold particles can be observed between the Arabidopsis wild-type Col-0 (A), the pad 2-1 mutant (B), the complemented line OE3 (C), and Col-0 after the treatment with 2mM buthionine sulfoximine (BSO) for 48 h (D). Note that cells of the pad 2-1 mutant (B) show slightly elevated levels of glutamate in most cell compartments, whereas the complemented line (C) and Col-0 plants treated with 2mM BSO for 48 h show increased levels only in mitochondria (M) and in chloroplasts and the cytosol, respectively, when compared to Col-0 (A). Inset in A shows gold particles bound to glutamate along the membranes of the endoplasmic reticulum (arrows). C, chloroplasts; CW, cell walls; IS, intercellular spaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles. Sections were post stained with uranyl acetate for 15 s. Bars: 1 μm and 0.5 μm in inset.
Figure 4.
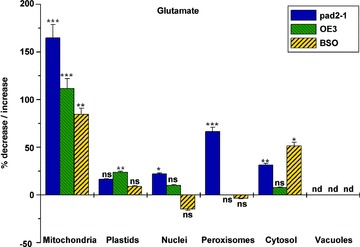
Quantitative analysis of glutamate specific labeling in the glutathione deficient mutant pad2-1, the complemented line OE3 and after the treatment of wildtype plants with buthionine sulfoximine (BSO). Graph shows means with standard errors and documents changes in the density of gold particles bound to glutamate in the respective Arabidopsis leaf cells when compared to control wildtype plants. Significant differences were calculated using the Mann-Whitney U-test; (*), (**), and (***), respectively, indicate significance at the 0.05, 0.01, 0.001 level of confidence. P > 0.05 was considered as not significant (ns); n > 20 for peroxisomes and vacuoles and n > 60 for all other cell structures. nd, not detected.
γ-EC
In wild type plants (Col-0), the greatest level of labeling density of γ-EC was detected in nuclei, which did not significantly differ from γ-EC-specific labeling in mitochondria, plastids, nuclei, peroxisomes and the cytosol (Table 1). Lowest levels of γ-EC were detected in vacuoles and γ-EC labeling was absent in cell walls and intercellular spaces in wild type plants (Figure 5). Compartment-specific labeling of γ-EC was strongly decreased in all cell compartments of the pad2-1 mutant and remained unchanged in all cell compartments of the complemented line OE3 when labeling density was compared to the wild type. In the pad2-1 mutant, the strongest decrease of γ-EC was observed in peroxisomes (–88%) followed by plastids (–82), nuclei (–79%), mitochondria (77), the cytosol (–74%) and vacuoles (–75%) when compared to the wild type (Figures 5,6). The treatment of wild type plants with BSO caused a strong decrease in γ-EC contents in all cell compartments between 91% and 98% in comparison to control wild type plants (Figure 6).
Figure 5.
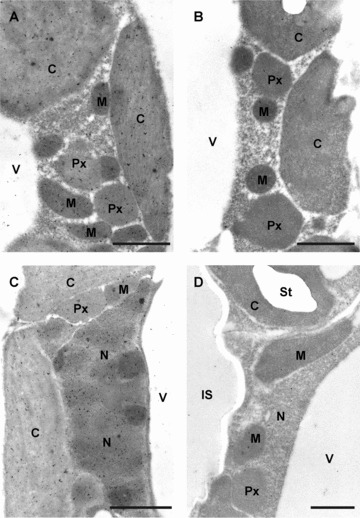
Transmission electron micrographs of mesophyll cells from Arabidopsis leaves after immunogold labeling of γ-glutamylcysteine (γ-EC). Different amounts of gold particles can be observed between the Arabidopsis wild-type Col-0 (A), the pad 2-1 mutant (B), the complemented line OE3 (C), and Col-0 after the treatment with 2mM buthionine sulfoximine (BSO) for 48 h (D). Note that cells of the OE3 line (C) show similar amounts of gold particles bound to γ-EC in all organelles, whereas the pad 2-1 mutant (B) and wild type plants treated with BSO (D) contain much lower amounts of gold particles bound to γ-EC in all cell compartments when compared to Col-0 (A). C, chloroplasts; CW, cell walls; IS, intercellular spaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles. Sections were post stained with uranyl acetate for 15 s. Bars: 1 μm.
Figure 6.
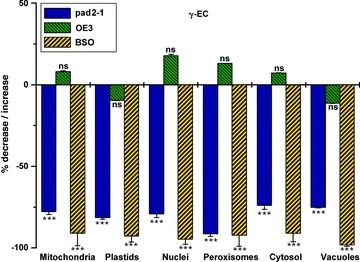
Quantitative analysis of γ-glutamylcysteine (γ-EC)specific labeling in the glutathione deficient mutant pad2-1, the complemented line OE3 and after the treatment of wild type plants with buthionine sulfoximine (BSO). Graph shows means with standard errors and documents changes in the density of gold particles bound to γ-EC in the respective Arabidopsis leaf cells when compared to control wildtype plants. Significant differences were calculated using the Mann-Whitney U-test; (***) indicates significance at the 0.001 level of confidence. P > 0.05 was considered as not significant (ns); n > 20 for peroxisomes and vacuoles and n > 60 for all other cell structures.
Glycine
In wild type plants (Col-0), the greatest level of glycine-specific labeling was detected in the cytosol. Intermediate labeling in this accession was found in mitochondria, nuclei and peroxisomes, which contained significantly less glycine than the cytosol (Table 1). The lowest glycine-specific labeling intensity was detected in plastids and vacuoles (Figure 7). A strong increase in glycine-specific labeling was observed in most cell compartments of the pad2-1 mutant when compared to the wild type, whereas in the complemented line OE3 unchanged levels were found in most cell compartments. In the pad2-1 mutant, the strongest increase in glycine-specific labeling was detected in nuclei (95%), followed by mitochondria (67%), the cytosol (37%) and peroxisomes (24%) when compared to wild type plants. Whereas unchanged levels of glycine were found in plastids of the pad2-1 mutant, a decrease was found in vacuoles (−44%) in comparison to wild type plants. In the complemented lines, vacuoles showed a decrease in glycine-specific labeling (−44%), whereas glycine contents in the other cell compartments remained unchanged when glycine labeling was compared to the wild type (Figures 7,8). BSO-treatment of wild type plants caused a massive increase in glycine contents in all cell compartments when labeling levels were compared to control wild type plants. Glycine was increased in nuclei (137%), the cytosol (125%), peroxisomes (109%), mitochondria (82%) and plastids (54%). A decrease was observed in vacuoles (–47%; Figure 8).
Figure 7.
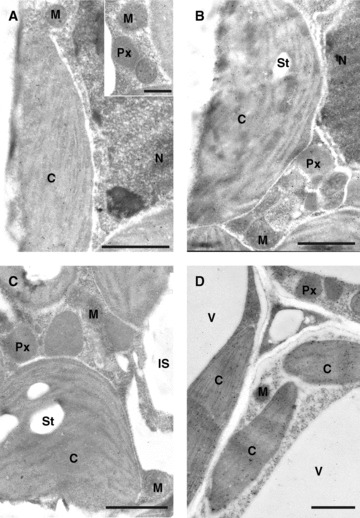
Transmission electron micrographs of mesophyll cells from Arabidopsis leaves after immunogold labeling of glycine. Different amounts of gold particles can be observed between the Arabidopsis wild-type Col-0 (A), the pad 2-1 mutant (B), the complemented line OE3 (C), and Col-0 after the treatment with 2 mM buthionine sulfoximine (BSO) for 48 h (D). Note that cells of the OE3 line (C) show similar amounts of gold particles bound to glycine whereas the pad 2-1 mutant (B) and wild type plants treated with BSO (D) contain higher amounts of gold particles bound to glycine in most cell compartments when compared to Col-0 (A). C, chloroplasts; CW, cell walls; IS, intercellular spaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles. Sections were post stained with uranyl acetate for 15 s. Bars: 1 μm.
Figure 8.
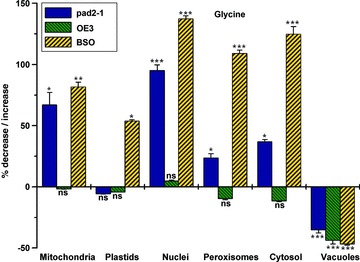
Quantitative analysis of glycine specific labeling in the glutathione deficient mutant pad2-1, the complemented line OE3 and after the treatment of wildtype plants with buthionine sulfoximine (BSO). Graph shows means with standard errors and documents changes in the density of gold particles bound to glycine in the respective Arabidopsis leaf cells when compared to control wildtype plants. Significant differences were calculated using the Mann-Whitney U-test; (*), (**), and (***), respectively, indicate significance at the 0.05, 0.01, 0.001 level of confidence. P > 0.05 was considered as not significant (ns); n > 20 for peroxisomes and vacuoles and n > 60 for all other cell structures.
Glutathione
The subcellular distribution of glutathione in Arabidopsis accession Col-0, the glutathione-deficient Arabidopsis mutant pad2-1 and the complemented line OE3 has been described previously in detail (Zechmann et al. 2008b). Nevertheless, parts of the data described previously in addition to data obtained during BSO treatment (2 mM for 48 h) have been included in this manuscript in order to allow a better correlation of changes in glutathione precursor contents with glutathione levels under these conditions. These results show that glutathione contents in leaves of the pad2-1 mutant were decreased when compared with wild type plants by about 92% in nuclei, 91% in peroxisomes, 86% in the cytosol, and 84% in plastids (Supplemental Figure 2). No significant change was found in mitochondria. In leaves of the complemented line OE3, gold particle density increased between 153% in peroxisomes, 125% in plastids, 78% in the cytosol, 52% in nuclei, and 43% in mitochondria in comparison to wild type. BSO-treatment of wild type plants significantly decreased glutathione contents in nuclei (–100%), peroxisomes (–100%), mitochondria (–96%), the cytosol (–92%) and in plastids (–76%) when compared with control wild type plants (Supplemental Figure 1).
Discussion
The subcellular distribution of glutathione precursors was studied by high resolution immuno electron microscopy in Arabidopsis plants. These studies revealed that all glutathione precursors were absent in the apoplast, but could be detected in different concentrations in the cytoplasm and nuclei of the cells (with the exception of glutamate in vacuoles). These results are similar to what has been found previously in pumpkin plants (Zechmann et al. 2006a, 2007b; Zechmann and Müller 2008) and extend the current knowledge of glutathione synthesis and degradation in plants.
The occurrence of glycine, cysteine and γ-EC, but not glutamate, in vacuoles of wild type plants indicates that glutathione could be degraded in two possible pathways within the vacuole and/or tonoplast. The first possible pathway for glutathione degradation within vacuoles could be triggered by GGT, which transfers glutamate to other dipeptides and leaves the dipeptide Cys-Gly, which could then be metabolized by a dipeptidase to its components (Foyer et al. 2001; Storozhenko et al. 2002; Shaw et al. 2005; Ohkama-Ohtsu et al. 2007a, 2007b). One isoform of GGT (GGT4 which was originally called GGT3 by Ohkama-Ohtsu et al. 2007a, 2007b but is refereed to GGT4 in the latest literature) has been detected within vacuoles (Ohkama-Ohtsu et al. 2007b; Ohkama-Ohtsu et al. 2008; Destro et al. 2011; Noctor et al. 2011) and could be responsible for the degradation of glutathione and glutathione-conjugates in this organelle.
The second possible pathway of glutathione degradation in vacuoles would involve the cleavage of glycine by carboxypeptidase (Steinkamp and Rennenberg 1985), which has been detected in vacuoles of tomato (Wolf et al. 1996) but still has yet to be established for Arabidopsis thaliana. Both of these reactions would leave cysteine, glycine and γ-EC in vacuoles. As glutamate would be transferred to other dipeptides it could not be detected with the method used in this study.
Glutathione and its precursors were absent in the apoplast of wild type plants, which indicates that glutathione in the apoplast of Arabidopsis thaliana is degraded very rapidly by GGT and that the degradation products are transported back into the cytosol. Two isoforms of the enzyme (GGT1 and GGT2) responsible for glutathione degradation in the apoplast have been found to be associated or bound to the plasmalemma in Arabidopsis thaliana (Ohkama-Ohtsu et al. 2007a; Destro et al. 2011). While GGT2 was detected only in young siliques (Ohkama-Ohtsu et al. 2007a) and roots (Destro et al. 2011), GGT1 activity was also detected in leaves. In light of these results, we propose that glutathione degradation in A. thaliana leaves is performed by GGT1 at the plasmalemma and that the resulting products (Cys-Gly and glutamate) are either further metabolized by dipeptidases or rapidly transported back into the cytoplasm. Within individual cells, glutathione precursors were found in all cell compartments in different concentrations.
Glutathione synthesis strongly depends on the availability of its precursors in glutathione synthesizing organelles such as plastids and the cytosol (Noctor et al. 2011) where glutathione precursor levels were present in intermediate concentrations in the accession Col-0. Very little is known about their subcellular distribution during impaired glutathione synthesis. In the present study we were able to demonstrate that the inhibition of the first enzyme of glutathione synthesis, either through BSO-treatment or in the GSH1 single point mutant pad2-1, led to the accumulation of cysteine, glycine and glutamate in most cell compartments including plastids and the cytosol when labeling intensity was compared to wild type plants. A decrease of γ-EC and glutathione was observed in all cell compartments. Expectantly, plastids, which are considered to be the main production center for γ-EC in Arabidopsis (Wachter et al. 2005; Noctor et al. 2011), showed a strong decrease (over 80%) of this glutathione precursor in the glutathione deficient pad2-1 mutant in comparison to wild type plants of accession Col-0. This observation could be correlated with a strong decrease also in all other cell compartments. A similar situation was observed when plants were treated with BSO, which specifically inhibits GSH1. Thus, we can conclude that low levels of γ-EC, induced by the inhibition of GSH1 in the pad2-1 mutant and during BSO-treatment, limit glutathione synthesis, thus leading to a strong decrease in total glutathione contents. The complemented line OE3, with restored glutathione synthesis and glutathione contents similar to the wild type (Parisy et al. 2007; Zechmann et al. 2008a), showed similar or slightly increased labeling density of cysteine, glutamate, γ-EC, and glycine in all cell compartments in comparison to wild type plants. These results demonstrate that the complementation of the pad2-1 mutant with GSH1 cDNA from the wild type restored not only glutathione contents, but also levels of glutathione precursors in most cell compartments. Additionally, we can conclude from these studies that the antibody methods used in this study specifically detect changes in the desired glutathione precursors as glutathione precursor contents could be detected in concentrations that correlated well with the expected concentrations due to the modulation experiments.
Summing up, we can conclude that low glutathione contents induced by the inhibition of GSH1 in the pad2-1 mutant and during BSO-treatment are caused by low γ-EC contents in most cell compartments including glutathione producing organelles such as chloroplasts and the cytosol. The distribution of glutathione precursors in A. thaliana accession Col-0 is limited to the protoplast, which indicates an effective degradation of glutathione by GGT in the apoplast and that the resulting amino acids are rapidly transported back into the cytosol.
Material and Methods
Plant material
The experiments in this study were performed with Arabidopsis thaliana (L.) Heynh. ecotype Columbia (Col-0), the pad 2-1 (phytoalexin deficient) mutant and one transgenic line (OE3) overexpressing GSH1. The pad 2-1 mutant is characterized as having a single point mutation in gene At4g23100 that encodes GSH1, which catalyses the first step of glutathione synthesis (Parisy et al. 2007). The single point mutation in pad2-1 is characterized by a single G to A nucleotide transition at position 1 697 from the start codon of the gene At4g23100. This mutation leads to the replacement of a serine by an asparagine residue at position 298 in the 522 amino-acid protein (Parisy et al. 2007). The transgenic line OE3, which is equivalent to line 3 in Parisy et al 2007, was genetically complemented by using cDNA from GSH1 of Arabidopsis and insertion into the pad2-1 mutant as described in detail by Parisy et al. 2007. While glutathione contents are strongly decreased in the pad2-1 mutant, they are restored in the complemented line and reach or exceed values of the wild type (Parisy et al. 2007; Zechmann et al. 2008b).
All plants were cultivated in growth chambers under defined conditions. After stratification for 4 days at 4 °C seeds of the Arabidopsis mutant pad2-1 were grown on soil in growth chambers with 14:10 h light: dark (L:D) photoperiod. Day and night temperatures were 22 °C and 18 °C, respectively, the relative humidity was 60% and the plants were kept at 100% relative soil water content. Light intensity varied between 110 and 140 μmol/m2 per s. Plants were kept in pots with soil and were watered adequately. Harvesting of Arabidopsis plants was performed 4 weeks after stratification. Therefore, the youngest fully developed rosette leaves were harvested 2 h after the onset of the light period. Leaves at this stage were approximately 2 cm in length and 0.7 cm in width. Immunocytochemical methods were used to determine the subcellular distribution of cysteine, glutamate, glycine, γ-EC and glutathione in leaves of Arabidopsis.
BSO treatment
Four-week-old Arabidopsis plants raised in soil were treated with 2 mM L-buthionine [S,R] sulfoximine (BSO; Sigma-Aldrich, St. Louis, MO, USA) dissolved in distilled water (pH 7.2) for 48 h. A control group was treated with the same solution without BSO. The BSO-solution was exchanged every 12 h. Treatment was carried out in plastic dishes (100 mL) covered with a nylon mesh by immersing the roots into the solutions. For treatment, seedlings were carefully removed from the pots filled with soil and the roots were gently rinsed with tap water (pH 7.2) until the soil was washed off. The roots were then immersed into the solution, whereas the stems and leaves were held by the nylon mesh above the solution.
Sample preparation for transmission electron microscopy and immunogold labeling
Preparation of samples for transmission electron microscopy (TEM) and immunogold labeling of glutathione was done with ultrathin sections on nickel grids as described in Zechmann et al. (2006a, 2006b, 2008b). Small samples of the youngest fully developed leaves (about 1.5 mm2) from at least three different plants were fixed in 2.5% paraformaldehyde/0.5% glutardialdehyde in 0.06M phosphate buffer (pH 7.2) for 90 min at room temperature (RT). Samples were then rinsed in buffer and dehydrated in increasing concentrations of acetone (50%, 70%, and 90%) at RT for 20 min at each step. Subsequently, specimens were gradually infiltrated with increasing concentrations of LR-White resin (30%, 60% and 100%; London Resin Company Ltd., Berkshire, UK) mixed with acetone (90%) and finally embedded in LR-White resin and polymerized at 50 °C for 48 h in small plastic containers.
Ultrathin sections (80 nm) of the samples were blocked with 2% bovine serum albumine (BSA, Sigma-Aldrich) in phosphate buffered saline (PBS, pH 7.2), then treated with the primary antibodies (anti-glutathione rabbit polyclonal IgG, anti-cysteine rabbit polyclonal IgG, anti-glutamate rabbit polyclonal IgG, anti-glycine rabbit polyclonal IgG, Millipore Corp., (Billerica, MA, USA); anti-γ-glutamylcysteine rabbit polyclonal IgG produced by Agrisera, Vännäs, Sweden) diluted 1:50 (anti-glycine and anti-γ-glutamylcysteine) and 1:300 (anti-cysteine and anti-glutamate) in PBS containing 1% goat serum for 2 h at RT. After a short rinse in PBS, samples were incubated with a 10 nm gold-conjugated secondary antibody (goat anti-rabbit IgG, British BioCell International, Cardiff, UK) diluted 1:50 (anti-glutathione, anti-cysteine and anti- γ-glutamylcysteine) and 1:100 (anti-glutamate and anti-glycine) in PBS for 90 min at RT. After a short wash in PBS, and distilled water labeled grids were either immediately observed under a Philips CM10 transmission electron microscope or post-stained with uranyl-acetate (15 s).
The specificity of the immunogold labeling procedures was tested by several negative controls. Negative controls were treated either with: (i) pre-immune serum instead of the primary antibody; (ii) gold conjugated secondary antibody (goat anti-rabbit IgG) without the primary antibody; (iii) non-specific secondary antibody (goat anti-mouse IgG); and (iv) primary antibodies pre-absorbed with an excess of either glutathione, cysteine, glutamate, glycine or γ-EC for 2 h at RT prior to labeling of the sections. For the latter solutions containing either 10 mM of glutathione, cysteine, glutamic acid, glycine or γ-EC were incubated with 0.5% glutardialdehyde for one hour. The excess of glutardialdehyde was then saturated by incubation for 30 min in a solution of 1% (w/v) BSA. The resulting solutions were then used to saturate the respective antibodies for 2 h prior to its use.
Negative controls for cysteine (Supplemental Figure 2), glutamate (Supplemental Figure 3), γ-EC (Supplemental Figure 4) and glycine (Supplemental Figure 5) revealed that no labeling occurred on the section when they were treated with (i) pre-immune serum instead of the primary antibody (image a in Supplemental Figures 2–5); (ii) gold conjugated secondary antibody without the primary antibody (image b in Supplemental Figures 2–5); (iii) non-specific secondary antibody (image c in Supplemental Figures 2–5); and (iv) primary antibodies pre-absorbed with an excess of either cysteine, glutamic acid, glycine or γ-EC for 2 h at RT prior to labeling of the sections (image d in Supplemental Figures 2–5). Negative controls for the glutathione antibody have been published previously (Zechmann et al. 2005, 2008b; Zechmann and Müller 2010).
In order to test if the antibodies against cysteine and glycine also bind to the dipeptide cys-gly the antibodies were incubated with a 10 mM cys-gly solution (Sigma-Aldrich) for 2 h. Subsequently the antibodies were then applied on sections of the wild type. Labeling results revealed that the pre-incubation of the cysteine, and glycine antibodies with cys-gly (Supplemental Figure 6) did not induce changes in cysteine and glycine labeling as gold particle density was similar to what was observed in wild type without the pre-incubation of the antibodies with cys-gly (Figures 1A,7A). Thus, these results demonstrate that the antibodies against cysteine and glycine do not bind to the dipeptide cys-gly, otherwise labeling would have been strongly decreased after the incubation as cys-gly would have bound and subsequently blocked the binding sites of the antibodies.
Micrographs of randomly photographed immunogold-labeled sections were digitized and gold particles were counted automatically using the software package Cell D with the particle analysis tool (Olympus, Life and Material Science Europa GmbH, Hamburg, Germany) in manually identified cell structures. For statistical evaluation, at least four different samples were examined for each treatment or mutant. From these samples a minimum of 20 (peroxisomes and vacuoles) to 60 (other cell structures) sectioned cell structures of at least 15 different cells were analyzed for gold particle density per treatment. The obtained data were statistically evaluated using Statistica (Stat-Soft Europe, Hamburg, Germany). The obtained data were presented as the number of gold particles per μm2. For statistical analyses either the Mann-Whitney U-test or the non-parametric Kruskal-Wallis test followed by a post-hoc comparison according to Conover was used. P < 0.05 was considered as significant (Bortz et al. 2008).
(Co-Editor: Katie Dehesh)
Acknowledgments
This work was supported by the Austrian Science Fund (FWF, P20619 and P22988 to B.Z.).
Supporting Information
Additional Supporting Information may be found in the online version of this article
Quantitative analysis ofglutathione specific labeling in the glutathione deficient mutantpad2-1, the complemented line OE3 and after thetreatment of wild type plants with BSO. Graph shows means withstandard errors and documents changes in the density of goldparticles bound to glutathione in the respective Arabidopsisleaf cells when compared to control wildtype plants. Significantdifferences were calculated using the Mann-Whitney U-test;(*), (**), and (***), respectively, indicate significance at the0.05, 0.01, 0.001 level of confidence. P > 0.05 wasconsidered as not significant (ns); n > 20 forperoxisomes and vacuoles and n > 60 for all other cell structures.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for cysteine labeling. Gold particles were absentwhen cells were treated with pre-imune serum instead of the primaryantibody (a), after the omission of the primary antibody (b), withan unspecific secondary antibody (c) and the cysteine antibodypre-absorpt with an excess of cysteine prior to its application(d). C, chloroplasts; CW, cell walls; IS, intercellular spaces; M,mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles.Sections were post stained with uranyl acetate for 15 s. Bars: 1μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for glutamate labeling. Gold particles were absentwhen cells were treated with pre-immune serum instead of theprimary antibody (a), after the omission of the primary antibody(b), with an unspecific secondary antibody (c) and the glutamateantibody pre-absorpt with an excess of glutamic acid prior to itsapplication (d). C, chloroplasts; CW, cell walls; IS, intercellularspaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V,vacuoles. Sections were post stained with uranyl acetate for 15 s.Bars: 1 μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for γ-EC labeling. Gold particles wereabsent when cells were treated with pre-imune serum instead of theprimary antibody (a), after the omission of the primary antibody(b), with an unspecific secondary antibody (c) and the γ-ECantibody pre-absorpt with an excess of γ-EC prior to itsapplication (d). C, chloroplasts; CW, cell walls; IS, intercellularspaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V,vacuoles. Sections were post stained with uranyl acetate for 15 s.Bars: 1 μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for glycine labeling. Gold particles were absentwhen cells were treated with pre-imune serum instead of the primaryantibody (a), after the omission of the primary antibody (b), withan unspecific secondary antibody (c) and the glycine antibodypre-absorpt with an excess of glycine prior to its application (d).C, chloroplasts; CW, cell walls; IS, intercellular spaces; M,mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles.Sections were post stained with uranyl acetate for 15 second. Bars:1μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated withantibodies against cysteine (a) and glycine (b) after incubation ofthe antibodies with a 10 mM cys-gly solution for 2 h prior to thelabeling experiment. Gold particle density was found to be similarin the different cell compartments when compared to the sections ofthe wildtype which were treated with antibodies against cysteine(Figure 1A) and glycine (Figure 7A) without pre-incubation ofcys-gly. C, chloroplasts; CW, cell walls; IS, intercellular spaces;M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V,vacuoles. Sections were post stained with uranyl acetate for 15 s.Bars: 1 μm and 0.5 μm in inset.
References
- Ammar WB, Mediouni C, Tray B, Ghorbel MH, Jemal F. Glutathione and phytochelatin contents in tomato plants exposed to cadmium. Biol. Plant. 2008;52:314–320. [Google Scholar]
- Bloem E, Riemenschneider A, Volker J, Papenbrock J, Schmidt A, Salac I, Haneklaus S, Schnug E. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J. Exp. Bot. 2004;55:2305–2312. doi: 10.1093/jxb/erh236. [DOI] [PubMed] [Google Scholar]
- Bloem E, Haneklaus S, Schnug E. Sulphur-induced resistance (SIR) - Sulphur fertilization as a sustainable strategy for keeping plants healthy.[Schwefel-induzierte Resistenz (SIR) - Schwefeldüngung als nachhaltige Strategie zur Gesunderhaltung von Pflanzen] J. Consum. Prot. Food Saf. 2007;2:7–12. [Google Scholar]
- Blum R, Beck A, Korfte A, Stengel A, Letzel T, Lendzian K, Grill E. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 2007;49:740–749. doi: 10.1111/j.1365-313X.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- Blum R, Meyer KC, Wünschmann J, Lendzian KJ, Grill E. Cytosolic action of phytochelatin synthase. Plant Physiol. 2010;153:159–169. doi: 10.1104/pp.109.149922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz J, Lienert GA, Bohenke K. Verteilungsfreie Methoden in der Biostatistik. Heidelberg: Springer; 2008. [Google Scholar]
- Cairns NG, Pasternak M, Wachter A, Cobbett CS, Meyer AJ. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006;141:446–455. doi: 10.1104/pp.106.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Farinati S, Maistri S, Furini A. How plants cope with cadmium: Staking all on metabolism and gene expression. J. Integr. Plant Biol. 2008;50:1268–1280. doi: 10.1111/j.1744-7909.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- Destro T, Prasad D, Martignago D, Bernet IL, Trentin AR, Renu IK, Ferretti M, Masi A. Compensatory expression and substrate inducibility of γ-glutamyl transferase GGT2 isoform in Arabidopsis thaliana. J. Exp. Bot. 2011;62:805–815. doi: 10.1093/jxb/erq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dučić T, Maksimović V, Radotić K. Oxalate oxidase and non-enzymatic compounds of the antioxidative system in young Serbian spruce plants exposed to cadmium stress. Arch. Biol. Sci. Belgrade. 2008;60:67–76. [Google Scholar]
- DeRidder BP, Goldsbrough PB. Organ-specific expression of glutathione S-transferases and the efficacy of herbicide safeners in Arabidopsis. Plant Physiol. 2006;140:167–175. doi: 10.1104/pp.105.067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Brazier-Hicks M, Dixon DP, Cummins I. Chemical Manipulation of antioxidant defenses in plants. Adv. Bot. Res. 2005;42:1–32. [Google Scholar]
- Ferretti M, Destro T, Tosatto SCE, La Rocca N, Rascio N, Masi A. Gamma-glutamyl transferase in the cell wall participates in extracellular glutathione salvage from the root apoplast. New Phytol. 2009;181:115–126. doi: 10.1111/j.1469-8137.2008.02653.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Theodoulou FL, Delrot S. The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci. 2001;6:486–492. doi: 10.1016/s1360-1385(01)02086-6. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation and photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox. Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Grzam A, Tennstedt P, Clemens S, Hell R, Meyer AJ. Vacuolar sequestration of glutathione S-conjugates outcompetes a possible degradation of the glutathione moiety by phytochelatin synthase. FEBS Lett. 2006;580:6384–6390. doi: 10.1016/j.febslet.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gullner G, Tóbiás I, Fodor J, Kömives T. Elevation of glutathione level and activation of glutathione-related enzymes affect virus infection in tobacco. Free Rad. Res. 1999;31:155–161. doi: 10.1080/10715769900301451. [DOI] [PubMed] [Google Scholar]
- Gullner G, Dodge AD. Accumulation of glutathione in pea leaf discs exposed to the photooxidative herbicides acifluorfen and 5-aminolevulinic acid. J. Plant Physiol. 2000;156:111–117. [Google Scholar]
- Harms K, von Ballmoos P, Brunold C, Hofgen R, Hesse H. Expression of a bacterial serine acetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J. 2000;22:335–343. doi: 10.1046/j.1365-313x.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- Hartmann TN, Fricker MD, Rennenberg H, Meyer AJ. Cell-specific measurements of cytosolic glutathione in poplar leaves. Plant Cell Environ. 2003;26:965–975. doi: 10.1046/j.1365-3040.2003.01031.x. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Hönicke P, Wirtz M, Hell R, Rennenberg H, Kopriva S. Regulation of sulphate assimilation by glutathione in poplars (Populus tremula X P. alba) of wild type and overexpressing γ-glutamylcysteine synthetase in the cytosol. J. Exp. Bot. 2004;55:837–845. doi: 10.1093/jxb/erh094. [DOI] [PubMed] [Google Scholar]
- Hell R, Bergmann L. γ-glutamylcysteine synthetase in higher plants: Catalytic properties and subcellular localisation. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- Hurd TR, Filipovska A, Costa NJ, Dahm CC, Murphy MP. Disulphide formation in mitochondrial protein thiols. Biochem. Soc. Trans. 2005a;33:1390–1393. doi: 10.1042/BST0331390. [DOI] [PubMed] [Google Scholar]
- Hurd TR, Costa NJ, Dahm CC, Beer SM, Brown ST, Filipovska A, Murphy MP. Glutathionylation of mitochondrial proteins. Antioxid. Redox. Signal. 2005b;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- Kopriva S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann. Bot. 2006;97:479–495. doi: 10.1093/aob/mcl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MN, Slovin JP. Purified γ-glutamyl transpeptidases from tomato exhibit high affinity for glutathione and glutathione S-conjugates. Plant Physiol. 2000;122:1417–1426. doi: 10.1104/pp.122.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan S, Foyer CH. Engineering and genetic approaches to modulating the glutathione network in plants. Physiol. Plant. 2006;126:382–397. [Google Scholar]
- Meyer AJ, Fricker MD. Control of demand-driven biosynthesis of glutathione in green Arabidopsis suspension culture cells. Plant Physiol. 2002;130:1927–1937. doi: 10.1104/pp.008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Tausz M, Grill D. Histochemical tracing of glutathione by fluorescence microscopy and image analysis system in living plant cells. In: Denke A, Dornisch K, Fleischmann F, Graßmann J, Heiser I, Hippeli S, Oßwald W, Schempp H, editors. Different Pathways through Life - Biochemical Aspects of Plant Biology and Medicine. Munich: Lincom Europa; 1999. pp. 189–197. [Google Scholar]
- Nocito FF, Espen L, Crema B, Cocucci M, Sacchi GA. Cadmium induces acidosis in maize root cells. New Phytol. 2008;179:700–711. doi: 10.1111/j.1469-8137.2008.02509.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Valadier MH, Roux Y, Foyer CH. Light-dependent modulation of foliar glutathione synthesis and associated amino acid metabolism in poplar overexpressing c-glutamylcysteine synthetase. Planta. 1997a;202:357–369. [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Valadier MH, Roux Y, Foyer CH. The role of glycine in determining the rate of glutathione synthesis in poplar. Possible implications for glutathione production during stress. Physiol. Plant. 1997b;100:225–263. [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Foyer CH. Manipulation of glutathione and amino acid biosynthesis in the chloroplast. Plant Physiol. 1998;118:471–482. doi: 10.1104/pp.118.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:229–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 2002;53/372:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH. Glutathione. The Arabidopsis Book. 2011;9:e0142. doi: 10.1199/tab.0142. doi: 10.1199/tab.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji M, Saito K. Molecular and biochemical analysis of serine acetyltransferase and cysteine synthase towards sulfur metabolic engineering in plants. Amino Acids. 2002;22:231–243. doi: 10.1007/s007260200011. [DOI] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N, Radwan S, Peterson A, Zhao P, Badr AF, Xiang C, Oliver DJ. Characterization of the extracellular c-glutamyl transpeptidases, GGT1 and GGT2, in Arabidopsis. Plant J. 2007a;49:865–877. doi: 10.1111/j.1365-313X.2006.03004.x. [DOI] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N, Zhao P, Xiang C, Oliver DJ. Glutathione conjugates in the vacuole are degraded by γ-glutamyl transpeptidase GGT3 in Arabidopsis. Plant J. 2007b;49:878–888. doi: 10.1111/j.1365-313X.2006.03005.x. [DOI] [PubMed] [Google Scholar]
- Ohkamu-Ohtsu N, Oikawa A, Zhao P, Xiang C, Saito K, Oliver DJ. A γ-glutamyl transpeptidase-independent pathway of glutathione catabolism to glutamate via 5-oxoproline in Arabidopsis. Plant Physiol. 2008;148:1603–1613. doi: 10.1104/pp.108.125716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak M, Lim B, Wirtz M, Hell R, Cobbett CS, Meyer A. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J. 2008;53:999–1012. doi: 10.1111/j.1365-313X.2007.03389.x. [DOI] [PubMed] [Google Scholar]
- Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. Identification of PAD2 as a c-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2007;49:159–172. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- Senda K, Ogawa K. Induction of PR-1 accumulation accompanied by runaway cell death in the lsd1 mutant of Arabidopsis is dependent on glutathione levels but independent of the redox state of glutathione. Plant Cell Physiol. 2004;45:1578–1585. doi: 10.1093/pcp/pch179. [DOI] [PubMed] [Google Scholar]
- Shaw ML, Pither-Joyce MD, McCallum JA. Purification and cloning of a γ-glutamyl transpeptidase from onion (Allium cepa. Phytochem. 2005;66:515–522. doi: 10.1016/j.phytochem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Steinkamp R, Rennenberg H. Degradation of glutathione in plant cells: Evidence against the participation of a γ-glutamyltranspeptidase. Z. Naturforsch. 1985;40:29–33. [Google Scholar]
- Storozhenko S, Belles-Boix E, Babiychuk E, Hérouart D, Davey MW, Slooten L, van Montagu M, Inzé D, Kushnir S. γ-glutamyl transpeptidase in transgenic tobacco plants. Cellular localization, processing, and biochemical properties. Plant Physiol. 2002;128:1109–1119. doi: 10.1104/pp.010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Nishimura J, Mochizuki Y, Inagaki K, Sekiya J. Homoglutathione synthesis in transgenic tobacco plants expressing soybean homoglutathione synthetase. Plant Biotechnol. 2004;21:79–83. [Google Scholar]
- Szalai G, Kellos T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009;28:66–80. [Google Scholar]
- Tausz M, Šircelj H, Grill D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004;55:1955–1962. doi: 10.1093/jxb/erh194. [DOI] [PubMed] [Google Scholar]
- Wachter A, Wolf S, Steininger H, Bogs J, Rausch T. Differential targeting of GSH1 and GSHS is achieved by multiple transcription initiation: Implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J. 2005;41:15–30. doi: 10.1111/j.1365-313X.2004.02269.x. [DOI] [PubMed] [Google Scholar]
- Wirtz M, Hell R. Dominant-negative modification reveals the regulatory function of the multimeric cysteine synthase protein complex in transgenic tobacco. Plant Cell. 2007;19:625–639. doi: 10.1105/tpc.106.043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AE, Dietz KJ, Schröder P. Degradation of glutathione S-conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett. 1996;384:31–34. doi: 10.1016/0014-5793(96)00272-4. [DOI] [PubMed] [Google Scholar]
- Zawoznik MS, Groppa MD, Tomaro ML, Benavides MP. Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Sci. 2007;173:190–197. [Google Scholar]
- Zechmann B, Mauch F, Sticher L, Müller M. Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. J. Exp. Bot. 2008b;59:4017–4027. doi: 10.1093/jxb/ern243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Müller M. Effects of zucchini yellow mosaic virus infection on the subcellular distribution of glutathione and its precursors in a highly tolerant Cucurbita pepo cultivar. Botany. 2008;86:1092–1100. [Google Scholar]
- Zechmann B, Müller M, Zellnig G. Intracellular adaptations of glutathione content in Cucurbita pepo (L.) induced by reduced glutathione and buthionine sulfoximine treatment. Protoplasma. 2006b;227:197–209. doi: 10.1007/s00709-005-0129-z. [DOI] [PubMed] [Google Scholar]
- Zechmann B, Müller M, Zellnig G. Modified levels of cysteine affect glutathione metabolism in plant cells. In: Khan NA, Singh S, Umar S, editors. Sulfur Assimilation and Abiotic Stress in Plants. Berlin, Heidelberg: Springer Verlag; 2008a. pp. 193–206. [Google Scholar]
- Zechmann B, Zellnig G, Müller M. Changes in the subcellular distribution of glutathione during virus infection in Cucurbita pepo (L.) Plant Biol. 2005;7:49–57. doi: 10.1055/s-2004-830477. [DOI] [PubMed] [Google Scholar]
- Zechmann B, Zellnig G, Müller M. Immunocytochemical localization of glutathione precursors in plant cells. J. Electron Microsc. 2006a;55:173–181. doi: 10.1093/jmicro/dfl022. [DOI] [PubMed] [Google Scholar]
- Zechmann B, Zellnig G, Müller M. Virus-induced changes in the subcellular distribution of glutathione precursors in Cucurbita pepo (L.) Plant Biol. 2007b;9:427–434. doi: 10.1055/s-2006-924670. [DOI] [PubMed] [Google Scholar]
- Zechmann B, Zellnig G, Urbanek-Krajnc A, Müller M. Artificial elevation of glutathione affects symptom development in ZYMV-infected Cucurbita pepo L. plants. Arch. Virol. 2007a;152:747–762. doi: 10.1007/s00705-006-0880-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitative analysis ofglutathione specific labeling in the glutathione deficient mutantpad2-1, the complemented line OE3 and after thetreatment of wild type plants with BSO. Graph shows means withstandard errors and documents changes in the density of goldparticles bound to glutathione in the respective Arabidopsisleaf cells when compared to control wildtype plants. Significantdifferences were calculated using the Mann-Whitney U-test;(*), (**), and (***), respectively, indicate significance at the0.05, 0.01, 0.001 level of confidence. P > 0.05 wasconsidered as not significant (ns); n > 20 forperoxisomes and vacuoles and n > 60 for all other cell structures.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for cysteine labeling. Gold particles were absentwhen cells were treated with pre-imune serum instead of the primaryantibody (a), after the omission of the primary antibody (b), withan unspecific secondary antibody (c) and the cysteine antibodypre-absorpt with an excess of cysteine prior to its application(d). C, chloroplasts; CW, cell walls; IS, intercellular spaces; M,mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles.Sections were post stained with uranyl acetate for 15 s. Bars: 1μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for glutamate labeling. Gold particles were absentwhen cells were treated with pre-immune serum instead of theprimary antibody (a), after the omission of the primary antibody(b), with an unspecific secondary antibody (c) and the glutamateantibody pre-absorpt with an excess of glutamic acid prior to itsapplication (d). C, chloroplasts; CW, cell walls; IS, intercellularspaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V,vacuoles. Sections were post stained with uranyl acetate for 15 s.Bars: 1 μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for γ-EC labeling. Gold particles wereabsent when cells were treated with pre-imune serum instead of theprimary antibody (a), after the omission of the primary antibody(b), with an unspecific secondary antibody (c) and the γ-ECantibody pre-absorpt with an excess of γ-EC prior to itsapplication (d). C, chloroplasts; CW, cell walls; IS, intercellularspaces; M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V,vacuoles. Sections were post stained with uranyl acetate for 15 s.Bars: 1 μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated asnegative control for glycine labeling. Gold particles were absentwhen cells were treated with pre-imune serum instead of the primaryantibody (a), after the omission of the primary antibody (b), withan unspecific secondary antibody (c) and the glycine antibodypre-absorpt with an excess of glycine prior to its application (d).C, chloroplasts; CW, cell walls; IS, intercellular spaces; M,mitochondria; N, nuclei; Px, peroxisomes; St, starch; V, vacuoles.Sections were post stained with uranyl acetate for 15 second. Bars:1μm.
Transmission electron micrographsof mesophyll cells from Arabidopsis leaves treated withantibodies against cysteine (a) and glycine (b) after incubation ofthe antibodies with a 10 mM cys-gly solution for 2 h prior to thelabeling experiment. Gold particle density was found to be similarin the different cell compartments when compared to the sections ofthe wildtype which were treated with antibodies against cysteine(Figure 1A) and glycine (Figure 7A) without pre-incubation ofcys-gly. C, chloroplasts; CW, cell walls; IS, intercellular spaces;M, mitochondria; N, nuclei; Px, peroxisomes; St, starch; V,vacuoles. Sections were post stained with uranyl acetate for 15 s.Bars: 1 μm and 0.5 μm in inset.


