Abstract
Caspase-1 is known to activate the proinflammatory cytokines IL-1β and IL-18. Additionally, it can cleave other substrates, including proteins involved in metabolism. Recently, we showed that caspase-1 deficiency in mice strongly reduces high-fat diet-induced weight gain, at least partly caused by an increased energy production. Increased feces secretion by caspase-1-deficient mice suggests that lipid malabsorption possibly further reduces adipose tissue mass. In this study we investigated whether caspase-1 plays a role in triglyceride-(TG)-rich lipoprotein metabolism using caspase-1-deficient and wild-type mice. Caspase-1 deficiency reduced the postprandial TG response to an oral lipid load, whereas TG-derived fatty acid (FA) uptake by peripheral tissues was not affected, demonstrated by unaltered kinetics of [3H]TG-labeled very low-density lipoprotein (VLDL)-like emulsion particles. An oral gavage of [3H]TG-containing olive oil revealed that caspase-1 deficiency reduced TG absorption and subsequent uptake of TG-derived FA in liver, muscle, and adipose tissue. Similarly, despite an elevated hepatic TG content, caspase-1 deficiency reduced hepatic VLDL-TG production. Intestinal and hepatic gene expression analysis revealed that caspase-1 deficiency did not affect FA oxidation or FA uptake but rather reduced intracellular FA transport, thereby limiting lipid availability for the assembly and secretion of TG-rich lipoproteins. The current study reveals a novel function for caspase-1, or caspase-1-cleaved substrates, in controlling intestinal TG absorption and hepatic TG secretion.
Keywords: caspase-1, chylomicron, intestine, lipid absorption, liver, triglyceride metabolism, VLDL
Obesity is accompanied by low-grade chronic systemic inflammation characterized by increased circulating levels of proinflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 (1). Activation of inflammatory pathways induces metabolic disturbances, leading to insulin resistance, dyslipidemia, and cardiovascular diseases. Caspase-1 is a cysteine protease that is known for its crucial role in the activation of the proinflammatory cytokines IL-18 and IL-1β. Additionally, caspase-1 has been shown to cleave other substrates, including proteins involved in energy metabolism (2–4). Caspase-1 activation is controlled by the inflammasome, a multiprotein complex consisting of a member of the Nod-like receptor family (e.g., NLRP3), and the inflammasome adaptor molecule ASC (5). A close interaction has been described between the innate immune system and lipoprotein metabolism in general and in triglyceride (TG) metabolism in particular (6). This interaction has largely been derived from studies that evaluated the effects of lipopolysaccharide (LPS), a major component of the cell wall of gram-negative bacteria, and individual cytokines on lipoprotein metabolism (7–9).
Although inflammasome-mediated caspase-1 activity has recently been linked to metabolic disturbances such as steatohepatitis (10) and cardiovascular diseases (11), the role of caspase-1 in lipoprotein metabolism has never been elucidated. We recently showed that the absence of caspase-1 in mice is accompanied by a profound decrease in adipocyte size and total adipose tissue mass upon high-fat-diet (HFD)-induced obesity (12). Inhibition of caspase-1 in adipocytes in vitro does not limit adipocyte differentiation (12), suggesting that the decrease in adipose tissue mass in vivo is not due to a primary defect in adipogenesis but may be the result of a lack of sufficient lipid supply.
An increased energy expenditure may at least partly explain the marked reduction in adipose tissue that was observed in caspase-1-deficient (caspase-1−/−) mice (13). Besides energy expenditure, dietary intake and subsequent intestinal absorption of fat strongly contribute to the supply of lipids for storage in adipose tissue. Dietary fat is extremely efficiently absorbed in the small intestine and incorporated into chylomicrons for distribution to other tissues. Interestingly, caspase-1−/− mice fed a HFD display an increased feces weight (13), suggesting a decreased intestinal capacity for the absorption of dietary fat. We therefore hypothesized that caspase-1 might play a direct role in the regulation of (postprandial) TG-rich lipoprotein metabolism that could contribute to the reduction in adipose tissue mass in caspase-1−/− mice.
In the current study, we investigate the role of caspase-1 in TG metabolism and show that caspase-1 deficiency in mice markedly reduces intestinal TG absorption as well as hepatic very low-density lipoprotein (VLDL)-TG production, thereby limiting the availability of lipids for peripheral storage.
MATERIALS AND METHODS
Animals
Caspase-1−/− mice (12) were backcrossed 10 generations to C57Bl/6 mice, and age-matched wild-type (WT) C57Bl/6J mice were used as controls. Mice were housed under standard conditions with a 12-h light-dark cycle and were fed a standard mouse chow diet with free access to water. Experiments were performed in 14- to 16-week-old animals. When indicated, mice were fasted overnight (from 1800 h to 0800 h) or for 4 h (from 0800 h to 1200 h). All experiments were approved by the institutional ethical committee on animal care and experimentation of the Leiden University Medical Center.
Lipid composition of feces
Mice were individually housed for 4 days to determine food intake and collect feces quantitatively. Feces were weighed, freeze-dried, and ground, and fecal fatty acids (FA) were derivatized by methyl esterification. Therefore, 2 ml methanol/hexane (4:1 v/v) containing 80 µg pentadecanoic acid (C15:0) as an internal standard (Fluka) was added to 15 mg of feces. Then, 200 µL acetyl chloride (Merck) was added, and samples were incubated at 95°C. After cooling to 4°C, 5 ml 6% K2CO3 (Sigma) was added, and samples were centrifuged (10 min; 4,000 rpm, 4°C). The upper hexane layer was isolated and used for GC analysis of FA methyl esters (FAME). FAME were separated on a 50 m × 0.25 mm capillary GC column (CP Sil 88; Agilent technologies) in a 3800 GC gas chromatograph (Varian) equipped with a flame ionization detector. The injector and flame ionization detector were kept at 270°C. The column temperature was programmed from 170°C to 210°C. FAME were introduced by split injection (split ratio 20:1). Quantification was based on the area ratio of the individual FA to the internal standard.
To extract total cholesterol from feces, dried feces (10 mg) were incubated in 1 ml alkaline methanol (CH3OH: 1 M NaOH = 3:1 [v/v]) for 2 h at 80°C in screw-capped tubes using 5α-cholestane as internal standard. Tubes were cooled to room temperature, and the cholesterol was extracted twice with 2 ml petroleum ether. The combined petroleum ether layers were evaporated to dryness, and the cholesterol was silylated by DMF-Silprep (Alltech). Analysis was performed by GC using the 25 m×0.25 mm capillary GC column (CP Sil 5B; Chrompack International) in a 3800 GC gas chromatograph (Varian) equipped with a flame ionization detector. Quantitation was based on the area ratio of the cholesterol to the internal standard 5α-cholestane. Phospholipid concentration was determined after lipid extraction using a commercially available enzymatic kit from Roche Molecular Biochemicals (Indianapolis, IN).
Postprandial TG response
To measure the postprandial response, overnight-fasted mice received an intragastric load of 200 µL olive oil (Carbonell, Cordoba, Spain). Blood samples were drawn before (t = 0) and 1, 2, 4, and 8 h after the bolus into chilled capillaries coated with paraoxon (Sigma, St. Louis, MO) to prevent ongoing lipolysis (14). Plasma was assayed for TG with the commercially available enzymatic kit from Roche Molecular Biochemicals (Indianapolis, IN) and for free FA (FFA) using NEFA-C kit from Wako Diagnostics (Instruchemie, Delfzijl, The Netherlands).
In vivo clearance of TG-rich emulsion particles
VLDL-like TG-rich emulsion particles (80 nm) labeled with glycerol tri[3H]oleate [triolein (TO)] and [14C]cholesteryl oleate (CO) were prepared and characterized as described previously (15). To study the in vivo clearance of the VLDL-like TG-rich particles, mice were fasted for 4 h and injected (t = 0) via the tail vein with 200 µL of emulsion particles (1.0 mg TG per mouse). Blood samples were taken from the tail vein at 2, 5, 10, and 15 min after injection to determine the serum decay of [3H]TO and [14C]CO. Plasma volumes were calculated as 0.04706 × body weight (g) as determined from 125I-BSA clearance studies as described previously (16). After taking the last blood sample, the liver, hindlimb muscle, and brown adipose tissue (BAT) and the gonadal (gWAT), subcutaneous (sWAT) and visceral (vWAT) white adipose tissues were collected. Organs were dissolved overnight at 60°C in Tissue Solubilizer (Amersham Biosciences, Rosendaal, The Netherlands), and 3H and 14C activity was quantified. Uptake of [3H]TO- and [14C]CO-derived radioactivity by the organs was corrected for plasma radioactivity present in the respective tissues and expressed per mg wet tissue weight.
Intestinal TG absorption
To measure intestinal lipid absorption, overnight-fasted mice received an intragastric load of [ [3H]TO (5 µCi) (GE Healthcare, Little Chalfont, UK) in 200 µL olive oil (Carbonell, Cordoba, Spain). Blood samples were drawn before gavage (t = 0) and at 1 and 2 h after gavage, and serum 3H-activity was determined. Plasma volumes (ml) were calculated as 0.04706 × body weight (g) (16). Two hours after the oral lipid load, liver, muscle, BAT, gWAT, sWAT, and vWAT were collected. Organs were dissolved overnight at 60°C in Tissue Solubilizer (Amersham Biosciences, Rosendaal, The Netherlands), and was determined. In addition, the intestinal tract (i.e., duodenum, proximal jejunum, distal jenunum, and ileum) was isolated and washed twice in 10 ml PBS. Both the intestinal tissue and the nonabsorbed luminal content (in PBS) were examined for 3H-activity to determine the amount of absorbed versus nonabsorbed olive oil present in the intestinal tract.
In vivo hepatic VLDL-TG and VLDL-apoB production
To measure VLDL production in vivo, mice were fasted for 4 h and anesthetized by intraperitoneal injection of acepromazine (6.25 mg/kg Neurotranq; Alfasan International BV, Weesp, The Netherlands), midazolam (6.25 mg/kg Dormicum; Roche Diagnostics, Mijdrecht, The Netherlands), and fentanyl (0.31 mg/kg) (Janssen Pharmaceuticals, Tilburg, The Netherlands). Mice were injected intravenously with Tran[35S] label (150 µCi/mouse) (MP Biomedicals, Eindhoven, The Netherlands) to label newly produced apolipoprotein B (apoB). After 30 min at t = 0 min, Triton WR-1339 (Sigma-Aldrich) was injected intravenously (0.5 mg/g body weight, 10% solution in PBS) to block serum VLDL clearance. Blood samples were drawn before (t = 0) and at 15, 30, 60, and 90 min after injection and used for determination of plasma TG concentration as described above. After 120 min, mice were exsanguinated via the retro-orbital plexus. VLDL was isolated from serum after density gradient ultracentrifugation at d < 1.006 g/ml by aspiration (17) and examined for incorporated 35S-activity.
Liver lipids
Lipids were extracted from livers of mice that were in a fed state and after 4 h fasting according to a modified protocol from Bligh and Dyer (18). Briefly, a small piece of liver was homogenized in ice-cold methanol. After centrifugation, lipids were extracted by addition of 1800 µL CH3OH:CHCl3 (3:1 v/v) to 45 µL homogenate. The CHCl3 phase was dried and dissolved in 2% Triton X-100. Hepatic TG, total cholesterol (TC), and phospholipid concentrations were measured using commercial kits from Roche Molecular Biochemicals (Indianapolis, IN). Liver lipids were expressed per mg protein, which was determined using the BCA protein assay kit.
RNA isolation and qPCR analysis
Total RNA was obtained from the epithelial layer of the duodenum, jejunum, and ileum 2 h after an oral lipid bolus and from 4 h-fasted livers. RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), followed by DNase treatment and column purification using the RNeasy mini kit (Qiagen, Hilden, Germany). The RNA concentration was determined using a Nanodrop ND-1000 spectrophotometer, and cDNA analysis was done using iScript reagent (Biorad). qPCR analysis was carried out on a Bio-Rad MyIQ. 36B4 was used as housekeeping genes and PCR primer sequences were taken from the PrimerBank and ordered from Eurogentec (Seraing, Belgium). Sequences of the primers used are available upon request.
Plasma β-hydroxybutyrate
Plasma β-hydroxybutyrate was measured in 4 h-fasted plasma samples using the β-HB Assay Kit from Abcam (Cambridge, UK).
Western blot analysis
Tissues were homogenized by Ultraturrax (22,000 rpm; 2 × 5 s) in an ice-cold buffer (pH 7.4) containing 30 mM Tris HCl, 150 mM NaCl, 10 mM NaF, 1 mM EDTA, 1 mM Na3VO4, 0.5% (v/v) Triton X-100, 1% (v/v) SDS, and protease inhibitors (Complete, Roche, Mijdrecht, The Netherlands) at a 1:10 (w/v) ratio. Homogenates were centrifuged (13,000 rpm for 15 min at 4°C), and the protein content of the supernatant was determined using the BCA protein assay kit (Pierce, Rockford, IL). Proteins (20–50 µg) were separated by 7–10% SDS-PAGE followed by transfer to a polyvinylidene fluoride membrane. Membranes were blocked for 1 h at room temperature in TBS with Tween-20 with 5% nonfat dry milk followed by incubation overnight with the following antibodies: Actin (Sigma-Aldrich), MTP (BD Biosciences), and DGAT1 and SREBP1c (both from Novus Biologicals). Blots were then incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Bands were visualized by ECL and quantified using Image Lab.
Statistical analysis
Data are presented as means ± SD. Statistical significant differences were calculated using a Student's t-test (SPSS Inc., Chicago, IL). P < 0.05 was regarded statistically significant.
RESULTS
Absence of caspase-1 in mice reduces white adipose tissue mass
Caspase-1−/− and WT mice were evaluated for body weight and adipose tissue mass. In line with results from our previous study (12), caspase-1 deficiency markedly reduced white adipose tissue (WAT) mass, whereas body weight was similar between caspase-1−/− versus WT mice (30.0 ± 3.2 vs. 30.8 ± 2.5 g). The reduction in WAT was reflected by a reduction in the amount of gWAT (−69%; P < 0.001) and sWAT (−34%; P < 0.01) and a trend toward reduction in the amount of vWAT (−17%; P = 0.16). We have previously shown that caspase-1−/− mice have 62% increased bone mineral content, which likely explains why caspase-1 deficiency does not affect total body weight despite the reduced adipose tissue mass (12). No difference was observed for total lean weight (−3%; P = 0.51), liver weight (−7%; P = 0.12), or muscle weight (−9%; P = 0.27) between capase-1−/− and WT mice.
Absence of caspase-1 in mice reduces the postprandial response to an oral lipid load
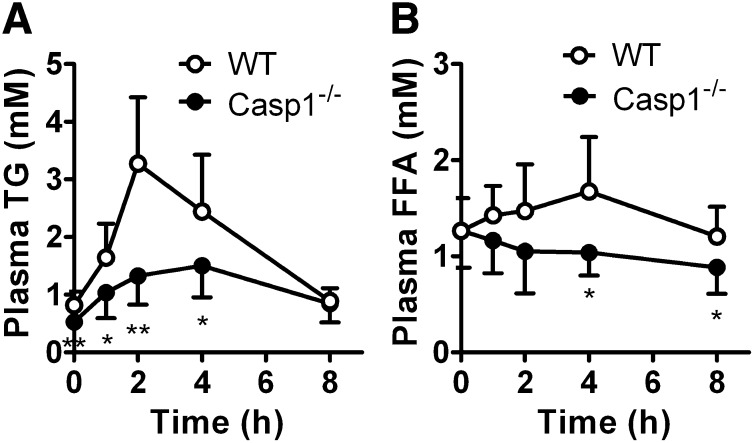
Because intestinal absorption of fat strongly contributes to the supply of lipids for storage in adipose tissue, we investigated whether the reduction in adipose tissue mass and the resistance to obesity upon high-fat feeding in caspase-1−/− mice (12, 13) reflects impaired postprandial lipid handling. Overnight-fasted WT and caspase-1−/− mice received an intragastric olive oil bolus (200 µL), and the appearance of TG and FFA in plasma was determined (Fig. 1). Basal plasma TG levels were reduced in caspase-1−/− mice compared with WT mice (0.53 ± 0.12 vs. 0.82 ± 0.24 mM; P < 0.01). Moreover, in caspase-1−/− mice, the postprandial increase in TG levels was markedly reduced (peak TG level −59% at t = 2 h; P < 0.01) (Fig. 1A), which was paralleled by a postprandial drop in serum FFA levels (peak FFA level −38% at t = 4 h; P < 0.05) (Fig. 1B).
Fig. 1.
Caspase-1 deficiency in mice reduces the postprandial lipid response. Overnight-fasted WT and caspase-1−/− mice received an intragastric olive oil gavage, and blood samples were drawn at the indicated time points. Plasma TG (A) and FFA (B) concentrations were determined in WT mice (open circles) and caspase-1−/− mice (closed circles). Values are means ± SD (n = 8). *P < 0.05; **P < 0.01.
Caspase-1 deficiency in mice does not affect TG-rich particle clearance
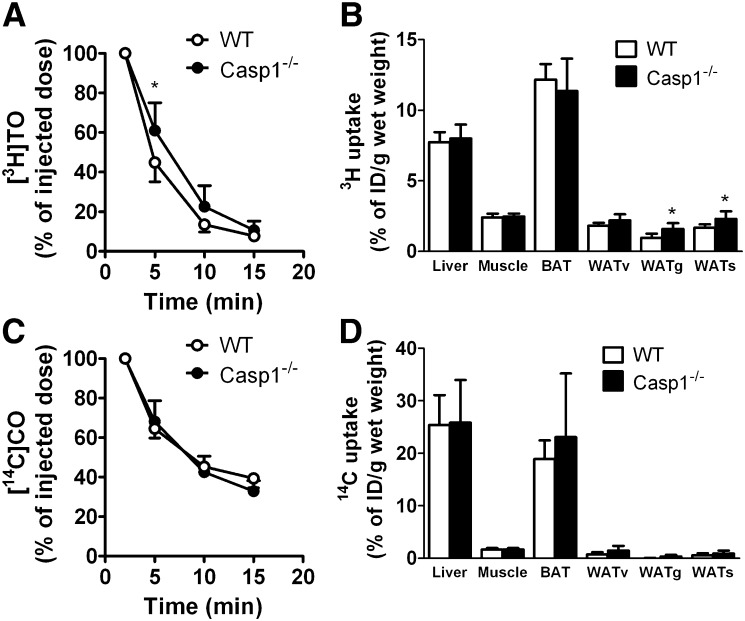
The postprandial TG response is determined by the balance between intestinal TG production and the rate of plasma TG clearance. To determine whether the decreased postprandial response in caspase-1−/− mice was caused by an increased clearance of TG-rich particles, we evaluated plasma clearance and organ distribution of [3H]TO and [14C]CO-double-labeled TG-rich emulsion particles in WT and caspase-1−/− mice (Fig. 2). Caspase-1 deficiency slightly delayed clearance of [3H]TG from plasma (Fig. 2A). However, at 15 min after the injection, WT and caspase-1−/− mice had equally cleared the [3H]TG from the circulation. Caspase-1 deficiency did not affect 3H uptake per g of tissue by the liver, muscle, BAT, and vWAT, whereas 3H uptake in gWAT and sWAT was increased in caspase-1−/− mice (Fig. 2B). This observation implies that the reduction in adiposity observed in caspase-1−/− mice cannot be explained by a defect in clearance and/or uptake of TG-derived [3H]FA by WAT. Similarly, caspase-1 deficiency did not affect [14C]CO clearance (Fig. 2C) or [14C]CO uptake by the various organs (Fig. 2D), indicating that TG-rich particle turnover was similar in caspase-1−/− and WT mice.
Fig. 2.
Caspase-1−/− mice have normal clearance of VLDL-like TG-rich particles. WT and caspase-1−/− mice were fasted for 4 h and received an intravenous bolus of [3H]TO and [14C]CO-double labeled TG-rich emulsion particles (1 mg TG per mouse) (n = 7–8). Blood was collected at the indicated time points, and 3H (A) and 14C (C) activities were determined in plasma of WT mice (open circles) and caspase-1−/− mice (closed circles). At 15 min after injection, organs were isolated, and uptake of 3H (B) and 14C (D) activity by the organs was measured. Values are means ± SD. *P < 0.05.
Absence of caspase-1 in mice reduces intestinal TG absorption and increases fecal FA content
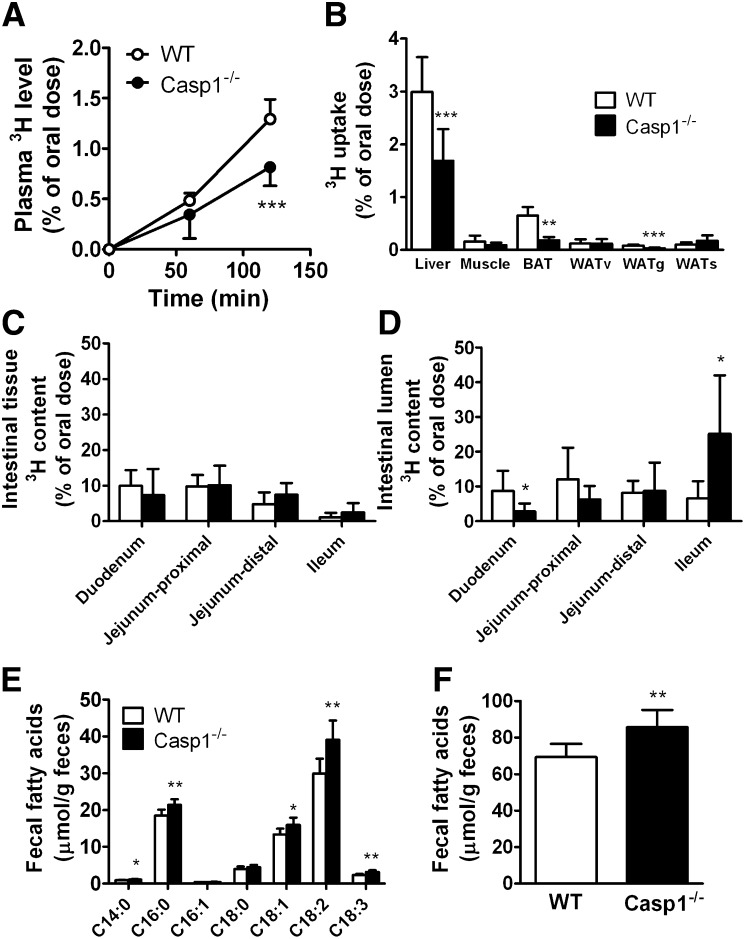
Because the markedly decreased postprandial TG response in caspase-1−/− mice was not explained by increased plasma TG clearance, we evaluated intestinal lipid absorption in caspase-1−/− and WT mice after an overnight fast. Mice were challenged with an oral lipid load containing 5 µCi of glycerol tri[3H]oleate in 200 µL olive oil followed by assessment of the appearance of 3H-activity in plasma and uptake of 3H-activity by tissues (Fig. 3). Two hours after the gavage, the appearance of 3H activity in plasma of caspase-1−/− mice was significantly reduced (−37%; P < 0.001) (Fig. 3A), suggesting a reduction in chylomicron-TG production. In line with this finding, a decreased amount of 3H activity was retrieved in the liver (−44%), muscle (−45%), BAT (−72%), and gWAT (−65%) of caspase-1−/− mice (Fig. 3B). Because we observed that caspase-1 deficiency does not affect clearance of TG-rich particles per se, these data confirm that caspase-1 deficiency reduces the intestinal TG absorption after an oral lipid load and as a consequence reduces uptake and incorporation of intestinally derived TG into peripheral lipid stores.
Fig. 3.
Caspase-1 deficiency in mice reduces intestinal TG production. Overnight-fasted WT and caspase-1−/− mice received an intragastric load of 200 µL olive oil containing [3H]triolein ([3H]TO) (n = 6–7). Blood samples were drawn at the indicated time points, and 3H activity was determined in plasma of WT mice (open circles) and caspase-1−/− mice (closed circles) (A). At 2 h after the oral lipid load, organs were collected, and uptake of 3H activity was measured (B). Intestine was isolated and washed twice to remove intestinal luminal nonabsorbed olive oil. 3H activity in the intestinal tissue (C) and the nonabsorbed 3H activity in the lumen (D) were determined. Feces was collected from individual housed WT and caspase-1−/− mice (n = 8), and fecal fatty acids were derivatized and extracted followed by fatty acid separation and quantification using gas chromatography. Individual fatty acids were determined (E), and total fatty acid content (µmol/g feces) was calculated from the sum of all individual fatty acids (F). Values are means ± SD. *P < 0.05; ***P < 0.001.
To determine whether the reduced intestinal TG absorption resulted in an increased amount of lipids remaining within the intestinal tract, we isolated the intestinal tract 2 h after the gavage and determined the amount of 3H that was present in the intestinal tissue and the nonabsorbed amount of 3H that was still present in the lumen of the intestinal tract. Caspase-1 deficiency did not affect the presence of [3H]TG-derived tissue-bound 3H activity in the different parts of the intestinal tract (Fig. 3C), suggesting sufficient intracellular lipid availability, which was confirmed by Oil red O staining (supplementary Fig. I). Instead, caspase-1 deficiency resulted in the accumulation of nonabsorbed [3H]TG in the lumen of the distal part of the intestinal tract (Fig. 3D), indicating malabsorption of TG. Microscopic analysis of the intestinal tract (duodenum, jejunum, and ileum) did not reveal any abnormalities (supplementary Fig. II).
To investigate whether the decrease in postprandial intestinal TG absorption results in long-term reduction of intestinal lipid absorption, feces were collected from individually housed mice over a 4-day period, and the fecal FA content was evaluated. Although food intake was similar between caspase-1−/− and WT mice (4.8 ± 0.5 vs. 5.1 ± 0.6 g/day), the feces of caspase-1−/− mice displayed an increased amount of C14:0, C16:0, C18:1, C18:2, and C18:3 compared with WT mice (Fig. 3E), resulting in an increase of the total fecal FA content (85.7 ± 9.4 vs. 69.4 ± 7.3 µmol/g feces; P < 0.01) (Fig. 3F). Fecal cholesterol and phospholipid levels were similar between caspase-1−/− and WT mice; therefore, caspase-1 deficiency specifically reduced the uptake of fatty acids because fecal cholesterol and phospholipid levels were similar between caspase-1−/− and WT mice (supplementary Fig. III).
Caspase-1 deficiency in mice decreases VLDL-TG production without affecting VLDL-apoB production despite increased hepatic lipid content
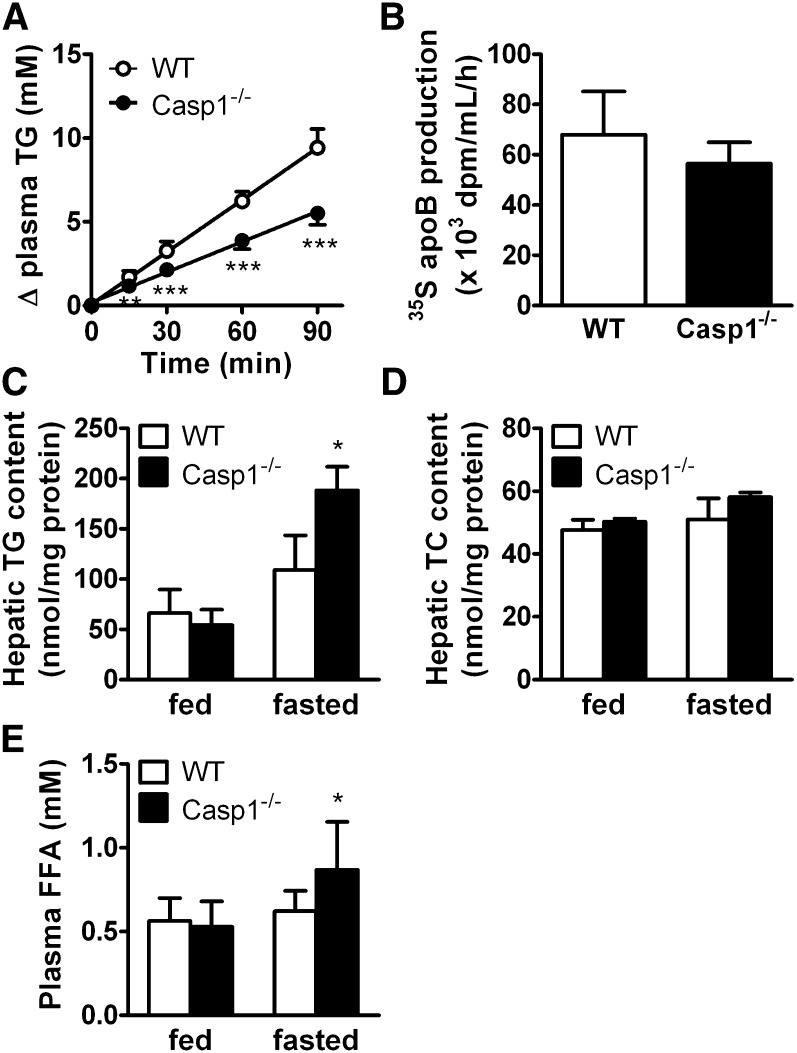
In fasting conditions, the availability of lipids for peripheral organs is determined by the production of VLDL-TG by the liver through mechanisms similar to intestinal chylomicron synthesis. Because caspase-1 deficiency reduces postprandial chylomicron-TG production, we hypothesized that caspase-1 deficiency could similarly affect hepatic VLDL-TG production. VLDL-TG production was measured in 4 h-fasted mice by determining plasma TG levels after Triton WR1339 injection (Fig. 4). Caspase-1−/− mice showed a strong reduction of TG accumulation in plasma compared with WT mice (Fig. 4A). The VLDL-TG production rate, as determined from the slope of the curve from all individual mice, was decreased by −42% (3.6 ± 0.5 vs. 6.3 ± 0.7 mM/h; P < 0.001), whereas the rate of VLDL-apoB production did not change significantly (Fig. 4B). Because the reduced rate of VLDL-TG production could be caused by a decreased hepatic lipid substrate availability in the liver, we evaluated the hepatic TG (Fig. 4C) and TC (Fig. 4D) content in livers from fed and 4 h-fasted WT and caspase-1−/− mice. In the fed state, hepatic lipid content was not affected by caspase-1 deficiency. Surprisingly, 4 h of fasting strongly increased hepatic TG content in caspase-1−/− compared with WT mice (+64%; P < 0.05). This may be the result of an increased FA influx from the plasma because plasma FFA levels were similarly increased after 4 h of fasting in caspase-1−/− compared with WT mice. These data suggest that caspase-1 deficiency is associated with reduced hepatic VLDL-TG secretion despite adequate availability of lipids from plasma and liver, suggesting a primary role of caspase-1 in the VLDL-TG secretion. In addition, the increased plasma FFA levels exclude a reduction in lipolytic response of adipose tissue as the underlying cause of the reduction in VLDL-TG production. Caspase-1 deficiency is thus associated with a reduction of intestinal chylomicron TG and hepatic VLDL-TG production, which may point toward a general role of caspase-1 in the assembly and/or secretion of TG-rich lipoprotein particles.
Fig. 4.
Caspase-1 deficiency in mice decreases VLDL-TG production without affecting VLDL-apoB production and increases hepatic steatosis. WT and caspase-1−/− mice were fasted for 4 h and received injections of Tran35S to label newly synthesized protein and Triton WR1339 to block lipolysis (n = 8). Blood samples were drawn at the indicated time points, and TG concentrations were determined in plasma of WT mice (open circles) and caspase-1−/− mice (closed circles) and plotted as the increase in plasma TG relative to t = 0 (A). TG production rates were determined by linear regression analysis. At 120 min, mice were exsanguinated, and VLDL was isolated and assayed for 35S-apoB (B). Livers were obtained from fed and 4 h-fasted WT and caspase-1−/− mice, and lipids were extracted (n = 3). Triglycerides (TG) (C) and total cholesterol (TC) (D) were measured and expressed per mg protein. Plasma FFA levels (E) were measured in plasma from fed and 4 h-fasted WT and caspase-1−/− mice. Values are means ± SD. *P < 0.05; ***P < 0.001.
Caspase-1 deficiency in mice reduces hepatic and intestinal expression of genes involved in lipogenesis
The secretion of intestinal chylomicron-TG in the postprandial state and hepatic VLDL-TG in the fasted state are regulated by similar pathways (19). Therefore, we further analyzed a selection of genes that are involved in the hepatic and intestinal secretion of TG (FA uptake, FA oxidation, lipogenesis, and VLDL/chylomicron secretion) in caspase-1−/− and WT mice by qPCR (Table 1). Liver samples were collected after 4 h of fasting, and intestinal samples were collected 2 h postprandial after an oral lipid load. Caspase-1−/− mice had normal expression levels of hepatic and intestinal apoB and microsomal TG transfer protein, which are involved in the assembly and secretion of chylomicrons and VLDL. Hepatic protein levels of MTP were not affected by caspase-1 deficiency (supplementary Fig. IV).
TABLE 1.
qPCR analysis of caspase-1−/− and wild-type fasted liver and postprandial intestinal samples reveals a global reduction in lipogenesis
| Intestine |
|||||||||
| Liver |
Duodenum |
Jejunum |
Ileum |
||||||
| Gene | Protein | Relative to WT liver |
Relative to WT duodenum |
||||||
| WT | Casp-1−/− | WT | Casp-1−/− | WT | Casp-1−/− | WT | Casp-1−/− | ||
| Fatty acid uptake and transport | |||||||||
| Slc27a4 | FATP4 | 1.00 ± 0.71 | 0.97 ± 0.76 | 1.00 ± 0.62 | 0.42 ± 0.19 | 1.05 ± 0.65 | 0.71 ± 0.31 | 0.31 ± 0.16 | 0.16 ± 0.09 |
| Cd36 | CD36 | 1.00 ± 0.74 | 0.32 ± 0.28 | 1.00 ± 0.41 | 2.07 ± 1.00* | 1.55 ± 0.90 | 3.37 ± 2.07 | 0.03 ± 0.06 | 0.06 ± 0.06 |
| Fabp1 | L-FABP | 1.00 ± 0.79 | 0.61 ± 0.54 | 1.00 ± 0.26 | 0.66 ± 0.20* | 0.75 ± 0.31 | 0.60 ± 0.43 | 0.01 ± 0.00 | 0.01 ± 0.01 |
| Fabp2 | I-FABP | 1.00 ± 0.72 | 0.53 ± 0.40 | 1.00 ± 0.23 | 0.80 ± 0.31 | 1.96 ± 1.52 | 0.98 ± 0.53 | 0.37 ± 0.37 | 0.21 ± 0.10 |
| Fatty acid oxidation | |||||||||
| Ppara | PPARα | 1.00 ± 0.85 | 1.05 ± 0.59 | 1.00 ± 0.43 | 2.34 ± 2.15 | 0.63 ± 0.25 | 1.42 ± 1.76 | 0.31 ± 0.17 | 0.34 ± 0.21 |
| Ppargc1a | PGC1α | 1.00 ± 0.84 | 1.68 ± 1.37 | 1.00 ± 0.44 | 0.83 ± 0.51 | 0.58 ± 0.24 | 1.47 ± 0.78* | 0.26 ± 0.09 | 0.25 ± 0.09 |
| Slc25A50 | CACT | 1.00 ± 0.96 | 1.09 ± 0.57 | 1.00 ± 0.32 | 0.94 ± 0.29 | 0.72 ± 0.35 | 0.59 ± 0.35 | 0.77 ± 0.33 | 0.61 ± 0.34 |
| Cpt1 | CPT-I | 1.00 ± 0.79 | 1.35 ± 1.15 | 1.00 ± 0.37 | 1.28 ± 0.47 | 0.39 ± 0.10 | 0.31 ± 0.09 | 0.25 ± 0.12 | 0.26 ± 0.11 |
| Lipogenesis | |||||||||
| Srebf1 | SREBP1a/c | 1.00 ± 0.65 | 0.20 ± 0.16* | 1.00 ± 0.48 | 0.50 ± 0.16* | 1.11 ± 0.74 | 1.46 ± 0.79 | 0.07 ± 0.01 | 0.05 ± 0.02 |
| Srebf2 | SREBP2 | 1.00 ± 0.98 | 1.13 ± 1.03 | 1.00 ± 0.36 | 0.52 ± 0.11* | 0.91 ± 0.30 | 0.57 ± 0.24 | 0.92 ± 0.36 | 0.45 ± 0.16* |
| Dgat1 | DGAT1 | 1.00 ± 0.84 | 1.00 ± 0.73 | 1.00 ± 0.43 | 0.67 ± 0.26 | 1.18 ± 0.76 | 0.77 ± 0.33 | 0.25 ± 0.12 | 0.13 ± 0.06 |
| Dgat2 | DGAT2 | 1.00 ± 0.98 | 0.63 ± 0.46 | 1.00 ± 0.35 | 0.72 ± 0.27 | 0.67 ± 0.36 | 0.50 ± 0.53 | 0.06 ± 0.04 | 0.03 ± 0.02 |
| Mogat1 | MGAT1 | N.D. | N.D. | 1.00 ± 0.62 | 0.83 ± 0.30 | 1.24 ± 0.85 | 0.68 ± 0.28 | 0.28 ± 0.16 | 0.13 ± 0.09 |
| Fasn | FAS | 1.00 ± 1.18 | 1.54 ± 0.76 | 1.00 ± 0.58 | 0.69 ± 0.11 | 0.85 ± 0.27 | 0.49 ± 0.20* | 0.71 ± 0.17 | 0.75 ± 0.40 |
| Lipoprotein assembly | |||||||||
| Mttp | MTP | 1.00 ± 0.78 | 0.82 ± 0.63 | 1.00 ± 0.28 | 0.71 ± 0.14 | 0.92 ± 0.39 | 0.55 ± 0.35 | 0.09 ± 0.06 | 0.04 ± 0.02 |
| Apob | ApoB | 1.00 ± 0.42 | 1.12 ± 0.84 | 1.00 ± 0.33 | 1.02 ± 0.36 | 1.60 ± 1.00 | 1.81 ± 1.13 | 0.44 ± 0.20 | 0.28 ± 0.13 |
Livers (4 h fasted) and intestines (2 h postprandial) were isolated from wild-type (WT) and caspase-1−/−mice. mRNA was isolated and mRNA expression of the indicated genes was quantified by qPCR. Data are calculated as fold change as compared with livers or duodenum (for intestines) of WT mice. Values are means ± SD (n = 5–6). *P < 0.05 compared with WT. Apob, apolipoprotein B; Cd36, fatty acid translocase; Cpt1, carnitine palmitoyltransferase 1; Dgat, diglyceride acyltransferase; Fabp1, liver type fatty acid binding protein; Fabp2, intestinal fatty acid binding protein; Fasn, fatty acid synthase; Mogat, monoacylglycerol O-acyltransferase 1; Mttp, microsomal triglyceride transfer protein; N.D., not determined; Ppara, peroxisome proliferator activated receptor α Slc27a4, fatty acid transport protein 4; Slc25A50, mitochondrial carnitine/acylcarnitine translocase; Srebf, sterol-regulatory element binding protein. Bold values represent significantly different values between WT and Casp-1−/− mice (marked additionally with the star behind the expression value for caspase-1−/− mice).
Likewise, genes involved in FA oxidation, including peroxisome proliferator activated receptor α, PPAR-γ coactivator 1-β, carnitine palmitoyltransferase 1, and mitochondrial carnitine/acylcarnitine translocase, were not changed in caspase-1−/− mice. In line with this, caspase-1 deficiency did not affect plasma β-hydroxybutyrate levels (supplementary Fig. V), suggesting no change in hepatic FA oxidation or ketogenesis. Genes involved in FA uptake, apart from an increased intestinal expression of Cd36, were also largely unaffected. Rather, caspase-1 deficiency reduced expression of the main regulator of lipogenesis, sterol-regulatory element binding protein 1 (Srebf1), in liver (−80%; P < 0.05) and intestine (−50%; P < 0.05). A similar trend was observed for hepatic protein levels of SREBP1c (supplementary Fig. IV). Furthermore, caspase-1−/− mice showed reduced expression of other genes involved in lipogenesis in the intestine (Srebf2 and fatty acid synthase [Fasn]). A reduction in de novo lipogenesis may reduce the intracellular pool of newly synthesized lipids that are available for secretion in triglyceride-rich lipoproteins. Moreover, intestinal expression of liver fatty acid-binding protein (Fabp1) was reduced in caspase-1−/− mice (−34%; P < 0.05). Because L-FABP is involved in the intracellular FA trafficking and secretion of chylomicrons in the intestine (20, 21), a reduction in Fabp1 expression may reduce lipid provision to the endoplasmatic reticulum, where lipidation of apoB occurs in the formation of TG-rich lipoprotein. These data imply that caspase-1 deficiency may reduce secretion of TG-rich lipoproteins by decreasing intracellular FA transport.
DISCUSSION
Caspase-1 activity has recently been established as an important mediator of obesity and obesity-related insulin resistance because caspase-1−/− mice are protected against HFD-induced obesity and associated insulin resistance (12, 13). The observed increase in energy expenditure in previous studies may at least partly underlie the reduction in adipose tissue in caspase-1−/− mice by negatively influencing the energy balance (12). When fed a HFD, caspase-1−/− mice display an increased feces production (13), suggesting that an impaired postprandial lipid handling may contribute to the reduction in adipose tissue. Therefore, with the current study we set out to evaluate the role of caspase-1 in (postprandial) lipid metabolism.
We have now established a role for caspase-1, either direct or indirect, in the postprandial absorption of lipids by the intestine and secretion of VLDL-TG by the liver. Mice lacking caspase-1 showed reduced postprandial chylomicron-TG secretion as well as fasting VLDL-TG secretion, leading to a reduced availability of TG-derived FA for uptake by peripheral organs and increased fat excretion in the feces. These data provide an additional mechanistic explanation besides an increased energy expenditure (13) for the reduced adipose tissue mass observed in caspase-1−/− mice upon HFD feeding and suggest a novel function for caspase-1 in controlling intestinal and hepatic intracellular FA transport and TG-rich lipoprotein assembly and secretion.
Our observations demonstrate that caspase-1 deficiency in mice does not affect the uptake of TG-derived FA by adipose tissue per se by showing that adipose tissue is fully capable of taking up [3H]FA derived from TG-rich particles that are administered directly into the circulation. This, together with our observation that in vitro adipogenesis is enhanced rather than reduced in adipocytes in the absence of caspase-1 (12), suggests that adipose tissue mass in caspase-1−/− mice is not reduced as a result of an impaired adipose tissue functioning by means of FA uptake and adipogenesis; rather, data from the current study show that caspase-1 deficiency reduces TG-derived FA delivery to the adipose tissue secondary to a reduction in TG-rich lipoprotein secretion.
It is interesting to speculate how caspase-1 deficiency reduces hepatic and intestinal TG secretion. The secretion of TG-rich lipoproteins is dependent on TG availability, which can be derived from FA supply from the diet (intestine), from plasma (liver), or from de novo lipogenesis. The reduction in intestinal TG secretion is not likely explained by a reduction in the uptake of FA because caspase-1 deficiency did not reduce intestinal or hepatic expression of Cd36 and fatty acid transport protein, proteins involved in FA transport; rather, intestinal expression of Cd36 was increased in caspase-1−/− mice. This may be a compensatory reaction to the reduced chylomicron secretion because CD36 has shown to be essential for the production of chylomicrons (22). Similar to the intestine, the reduction in hepatic TG secretion does not seem to be the result of a reduction in lipid uptake, based on the observation that plasma FFA levels are increased in caspase-1−/− mice and hepatic genes involved in FA uptake are not changed.
It appears that caspase-1 deficiency is associated with reduced expression of the intestinal and hepatic lipogenic genes such as Srebf1, Srebf2, and Fasn, which are involved in the FA synthesis and intracellular resynthesis of TG for the secretion of lipid-rich lipoproteins. This could suggest that a reduction in de novo lipogenesis may underlie the reduced secretion of TG-rich lipoproteins in caspase-1−/− mice. Within this scope, it is surprising that the reduction in the de novo lipogenesis is not paralleled by a reduced intestinal and hepatic lipid content. Tracer studies and Oil Red O staining of intestinal sections revealed that enterocytes of caspase-1−/− and WT mice contained similar amount of lipids after an oral gavage of olive oil. Moreover, the hepatic TG levels were increased rather than reduced in caspase-1−/− mice. Within this context, the reduced expression of lipogenic genes that we observe may be a consequence rather than a cause of the reduced TG secretion. Other studies indeed observed a reduction in Srebf1 as a response to steatosis (23). Alternatively, the reduction in expression of genes involved in de novo lipogenesis may be the result of reduced plasma insulin levels in caspase-1−/− mice (12) because insulin induces hepatic Srebf1 expression (24).
Before incorporation into TG-rich lipoproteins, lipids need to be activated and transported toward the ER and Golgi system. Because the reduction in intestinal TG secretion in caspase-1−/− mice cannot be explained by a reduction of lipid availability within the enterocytes, it may be caused by a reduction in intracellular transport of lipids. We observed a decreased intestinal expression of L-FABP in caspase-1−/− mice, a protein that is involved in the activation and transport of FA toward the ER, as well as budding of prechylomicron transport vesicles (21, 25). Because L-FABP-deficient mice display a reduction in intestinal lipid trafficking (20), it is conceivable that a reduced L-FABP expression limits chylomicron secretion, provided that a reduced expression of L-FABP affected the FABP-mediated FA transport in our study. In the liver, we observed that VLDL-TG secretion is reduced without changes in hepatic apoB secretion, suggesting that caspase-1 deficiency indeed affects apoB lipidation and secretion of the lipoprotein particle. Future studies may be aimed at delineating the role of caspase-1 in absorption of specific type of FAs. In contrast to long-chain FAs, dietary medium-chain FAs are incorporated into chylomicrons only to a limited extent but rather enter the portal vein as FA (26). Because our current findings show that caspase-1 deficiency reduces lipidation of apoB and secretion of chylomicrons, caspase-1 deficiency is expected to reduce absorption of long-chain, but not short-chain, FAs.
Our current observations regarding the role of caspase-1 deficiency in postprandial lipid metabolism are shown in absence of acute or HFD-induced inflammation, processes that are known to induce inflammasome-mediated caspase-1 activity (10, 12). Besides absorption of nutrients, the intestinal tract is the first barrier in the defense against invading pathogens. Intestinal epithelial cells, including enterocytes, sense microbial products that are able to activate inflammasome-mediated caspase-1 (27–29). In addition, accumulating evidence shows that dietary nutrients can activate inflammatory pathways in the intestine (30). Hence, intestinal caspase-1 could be directly activated by lipids and other nutrients in the diet. Correspondingly, hepatic caspase-1 may be activated by the FA influx upon fasting, similarly to the increased FA influx upon high-fat feeding that is known to activate the inflammasome (10).
It remains to be established how caspase-1 deficiency affects intracellular FA metabolism within the enterocyte. Caspase-1 is known to process a number of cytokines, including pro-IL-1β and pro-IL-18, into the active form, and the absence of caspase-1 is associated with reduced levels of bioactive IL-1β and IL-18. To what extent these differences are involved in the reduced production of TG-rich lipoprotein secretion in caspase-1−/− mice remains to be determined. IL-1Ra−/− mice, characterized by IL-1 oversignaling, display reduced adiposity (31, 32). In line with this, IL-1R−/− mice, characterized by impaired IL-1 signaling, display increased adiposity (33). These previous findings suggest that a reduction in active IL-1β in caspase-1−/− mice would increase rather than reduce adipose tissue mass, raising doubts about the role of IL-1β in our observations. However, adiposity in IL-1Ra−/− and IL-1R−/− mice is not only affected by changes in IL-1β but also by changes in IL-1α and IL-1Ra signaling, which hampers interpretation on the direct role of IL-1β. An animal model specifically deficient for IL-1β may be more suitable to study the role of IL-1β in adiposity and lipid metabolism. To our knowledge, such studies have not been performed. Another possibility is that the effects of enhanced activity of IL-1β (IL-1Ra−/− mice) and the absence of IL-1β activity (caspase-1−/− mice) are similar. In other words, a narrow window of IL-1β activity levels is apparently needed to efficiently control metabolism.
Injection of proinflammatory IL-1β in rats has long been known to increase lipogenesis and hepatic secretion of TG-containing lipoproteins (9), suggesting that the absence of bioactive IL-1β in caspase-1−/− mice could be responsible for the reduction in secretion of TG-containing lipoproteins. Accordingly, a recent report revealed that IL-1β gene polymorphisms affect the postprandial TG response in humans (34). Subjects with the polymorphism that conveys higher IL-1β activity displayed an increased postprandial TG response. Our data suggest that these observations may thus be explained by an increased intestinal and/or hepatic TG production rather than by reduced lipoprotein lipase activity, which has been suggested as a possible mechanism for their observation (35). An IL-1β-mediated increase in intestinal TG secretion may contribute to the tendency toward higher abdominal obesity previously observed in these subjects (36). Similar to IL-1β, genetic variation in IL-18 has been associated with postprandial TG levels (37), suggesting that the absence of caspase-1 may reduce TG-rich lipoprotein production indirectly via these cytokines. Future studies may verify whether our current observations are mediated via IL-1β or IL-18. Another possibility is that caspase-1 affects lipid metabolism independent of IL-1β or IL-18 because, in addition to IL-1β and IL-18, caspase-1 has been shown to be able to directly cleave additional substrates (2, 38). These substrates include several key metabolic proteins, such as SIRT1 and PPARγ (3, 4). It is reasonable to postulate that additional substrates of caspase-1 play regulatory roles in intracellular lipid metabolism. Future studies will therefore need to identify new potential cleavage sites in proteins involved in intracellular FA transport and lipoprotein assembly that may contribute to the reduction in TG secretion in caspase-1−/− mice.
In conclusion, we show that caspase-1−/− mice show an attenuated intestinal chylomicron-TG production and hepatic VLDL-TG production, both of which limit the availability of FA for uptake by adipose tissue. The current study reveals a novel function for caspase-1, or caspase-1-derived substrates, in controlling intestinal TG absorption and TG-rich lipoprotein assembly and secretion. We anticipate that caspase-1 may be a novel therapeutic target for the treatment of obesity and obesity-related disorders such as cardiovascular disease.
Supplementary Material
Acknowledgments
The authors thank A.C.M. Pronk, C. van der Bent, E.J. Pieterman, and C.J.A. van der Wee-Pals for excellent technical assistance.
Footnotes
Abbreviations:
- BAT
- brown adipose tissue
- Cd36
- fatty acid translocase
- CO
- [14C]cholesteryl oleate
- FA
- fatty acid
- Fabp1
- liver type fatty acid binding protein
- FAME
- FA methyl esters
- Fasn
- fatty acid synthase
- gWAT
- gonadal white adipose tissue
- HFD
- high-fat diet
- IL-1β
- interleukin 1 beta
- IL-18
- interleukin 18
- LPS
- lipopolysaccharide
- Srebf
- sterol-regulatory element binding protein
- sWAT
- subcutaneous white adipose tissue
- TC
- total cholesterol
- TG
- triglyceride
- TNF-α
- tumor necrosis factor-α
- TO
- triolein
- VLDL
- very low-density lipoprotein
- vWAT
- visceral white adipose tissue
- WAT
- white adipose tissue
- WT
- wild-type
This work was supported by an Amylin Paul Langerhans grant from the European Foundation for the Study of Diabetes and the seventh framework program of the EU-funded “LipidomicNet” (202272), by the Center of Medical Systems Biology (CMSB) and the Netherlands Consortium for Systems Biology (NCSB) established by The Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (NGI/NWO) ( I.O.C.M.V.), by a grant from the Dutch Diabetes Research Foundation (R.S.), by a Vici grant from the Netherlands Organization for Scientific Research (M.G.N.), and by the Netherlands Heart Foundation grant 2009T038 (P.C.N.R.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Trayhurn P., Wood I. S. 2005. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem. Soc. Trans. 33: 1078–1081 [DOI] [PubMed] [Google Scholar]
- 2.Shen J., Yin Y., Mai J., Xiong X., Pansuria M., Liu J., Maley E., Saqib N. U., Wang H., Yang X. F. 2010. Caspase-1 recognizes extended cleavage sites in its natural substrates. Atherosclerosis. 210: 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalkiadaki A., Guarente L. 2012. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He F., Doucet J. A., Stephens J. M. 2008. Caspase-mediated degradation of PPARgamma proteins in adipocytes. Obesity (Silver Spring). 16: 1735–1741 [DOI] [PubMed] [Google Scholar]
- 5.Franchi L., Eigenbrod T., Munoz-Planillo R., Nunez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10: 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khovidhunkit W., Kim M. S., Memon R. A., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45: 1169–1196 [DOI] [PubMed] [Google Scholar]
- 7.Feingold K. R., Staprans I., Memon R. A., Moser A. H., Shigenaga J. K., Doerrler W., Dinarello C. A., Grunfeld C. 1992. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid Res. 33: 1765–1776 [PubMed] [Google Scholar]
- 8.Bartolome N., Arteta B., Martinez M. J., Chico Y., Ochoa B. 2008. Kupffer cell products and interleukin 1beta directly promote VLDL secretion and apoB mRNA up-regulation in rodent hepatocytes. Innate. Immun. 14: 255–266 [DOI] [PubMed] [Google Scholar]
- 9.Feingold K. R., Soued M., Adi S., Staprans I., Neese R., Shigenaga J., Doerrler W., Moser A., Dinarello C. A., Grunfeld C. 1991. Effect of interleukin-1 on lipid metabolism in the rat. Similarities to and differences from tumor necrosis factor. Arterioscler. Thromb. 11: 495–500 [DOI] [PubMed] [Google Scholar]
- 10.Csak T., Ganz M., Pespisa J., Kodys K., Dolganiuc A., Szabo G. 2011. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 54: 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nunez G., Schnurr M., et al. 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464: 1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stienstra R., Joosten L. A., Koenen T., van Tits B., van Diepen J. A., van den Berg S. A., Rensen P. C., Voshol P. J., Fantuzzi G., Hijmans A., et al. 2010. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 12: 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stienstra R., van Diepen J. A., Tack C. J., Zaki M. H., van de Veerdonk F. L., Perera D., Neale G. A., Hooiveld G. J., Hijmans A., Vroegrijk I., et al. 2011. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 108: 15324–15329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambon A., Hashimoto S. I., Brunzell J. D. 1993. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J. Lipid Res. 34: 1021–1028 [PubMed] [Google Scholar]
- 15.Rensen P. C., van Dijk M. C., Havenaar E. C., Bijsterbosch M. K., Kruijt J. K., van Berkel T. J. 1995. Selective liver targeting of antivirals by recombinant chylomicrons–a new therapeutic approach to hepatitis B. Nat. Med. 1: 221–225 [DOI] [PubMed] [Google Scholar]
- 16.Jong M. C., Rensen P. C., Dahlmans V. E., van der Boom H., van Berkel T. J., Havekes L. M. 2001. Apolipoprotein C–III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J. Lipid Res. 42: 1578–1585 [PubMed] [Google Scholar]
- 17.Redgrave T. G., Roberts D. C., West C. E. 1975. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal. Biochem. 65: 42–49 [DOI] [PubMed] [Google Scholar]
- 18.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 19.Xiao C., Hsieh J., Adeli K., Lewis G. F. 2011. Gut-liver interaction in triglyceride-rich lipoprotein metabolism. Am. J. Physiol. Endocrinol. Metab. 301: E429–E446 [DOI] [PubMed] [Google Scholar]
- 20.Newberry E. P., Xie Y., Kennedy S. M., Luo J., Davidson N. O. 2006. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 44: 1191–1205 [DOI] [PubMed] [Google Scholar]
- 21.Neeli I., Siddiqi S. A., Siddiqi S., Mahan J., Lagakos W. S., Binas B., Gheyi T., Storch J., Mansbach C. M. 2007. Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J. Biol. Chem. 282: 17974–17984 [DOI] [PubMed] [Google Scholar]
- 22.Drover V. A., Ajmal M., Nassir F., Davidson N. O., Nauli A. M., Sahoo D., Tso P., Abumrad N. A. 2005. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 115: 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjorkegren J., Beigneux A., Bergo M. O., Maher J. J., Young S. G. 2002. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J. Biol. Chem. 277: 5476–5483 [DOI] [PubMed] [Google Scholar]
- 24.Foretz M., Guichard C., Ferre P., Foufelle F. 1999. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. USA. 96: 12737–12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqi S., Saleem U., Abumrad N. A., Davidson N. O., Storch J., Siddiqi S. A., Mansbach C. M. 2010. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J. Lipid Res. 51: 1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swift L. L., Hill J. O., Peters J. C., Greene H. L. 1990. Medium-chain fatty acids: evidence for incorporation into chylomicron triglycerides in humans. Am. J. Clin. Nutr. 52: 834–836 [DOI] [PubMed] [Google Scholar]
- 27.Hooper L. V., Macpherson A. J. 2010. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10: 159–169 [DOI] [PubMed] [Google Scholar]
- 28.Zaki M. H., Lamkanfi M., Kanneganti T. D. 2011. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 32: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duerkop B. A., Vaishnava S., Hooper L. V. 2009. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 31: 368–376 [DOI] [PubMed] [Google Scholar]
- 30.Ji Y., Sakata Y., Tso P. 2011. Nutrient-induced inflammation in the intestine. Curr. Opin. Clin. Nutr. Metab. Care. 14: 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuki T., Horai R., Sudo K., Iwakura Y. 2003. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J. Exp. Med. 198: 877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somm E., Henrichot E., Pernin A., Juge-Aubry C. E., Muzzin P., Dayer J. M., Nicklin M. J., Meier C. A. 2005. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 54: 3503–3509 [DOI] [PubMed] [Google Scholar]
- 33.McGillicuddy F. C., Harford K. A., Reynolds C. M., Oliver E., Claessens M., Mills K. H., Roche H. M. 2011. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 60: 1688–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgado-Lista J., Garcia-Rios A., Perez-Martinez P., Solivera J., Yubero-Serrano E. M., Fuentes F., Parnell L. D., Shen J., Gomez P., Jimenez-Gomez Y., et al. 2011. Interleukin 1B variant -1473G/C (rs1143623) influences triglyceride and interleukin 6 metabolism. J. Clin. Endocrinol. Metab. 96: E816–E820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Netea M. G., Dinarello C. A. 2011. More than inflammation: interleukin-1beta polymorphisms and the lipid metabolism. J. Clin. Endocrinol. Metab. 96: 1279–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen J., Arnett D. K., Peacock J. M., Parnell L. D., Kraja A., Hixson J. E., Tsai M. Y., Lai C. Q., Kabagambe E. K., Straka R. J., et al. 2007. Interleukin1beta genetic polymorphisms interact with polyunsaturated fatty acids to modulate risk of the metabolic syndrome. J. Nutr. 137: 1846–1851 [DOI] [PubMed] [Google Scholar]
- 37.Smart M. C., Dedoussis G., Yiannakouris N., Grisoni M. L., Dror G. K., Yannakoulia M., Papoutsakis C., Louizou E., Mantzoros C. S., Melistas L., et al. 2011. Genetic variation within IL18 is associated with insulin levels, insulin resistance and postprandial measures. Nutr. Metab. Cardiovasc. Dis. 21: 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamkanfi M., Kanneganti T. D., van Damme P., van den Berghe T., Vanoverberghe I., Vandekerckhove J., Vandenabeele P., Gevaert K., Nunez G. 2008. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol. Cell. Proteomics. 7: 2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.