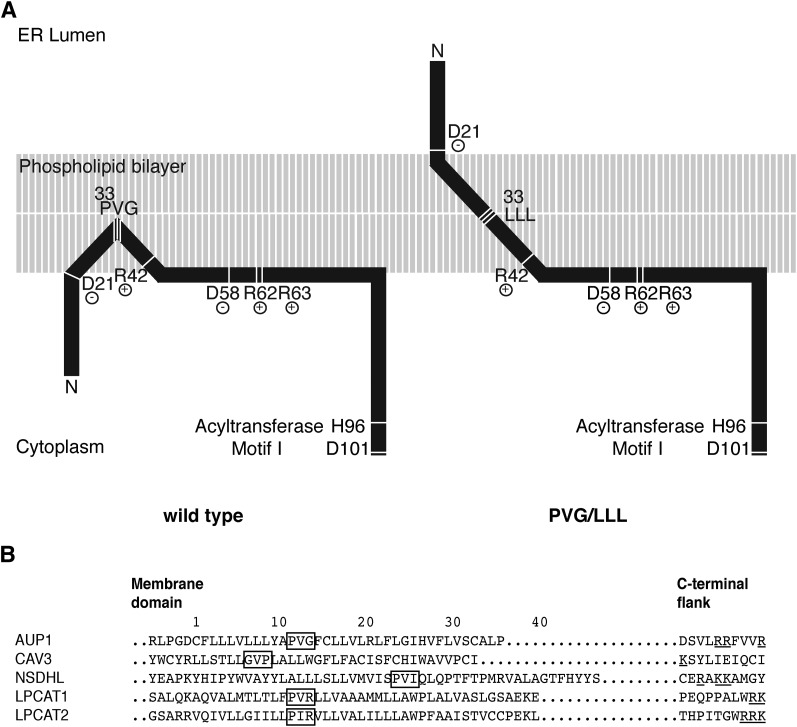
Fig. 8.
Model of the AUP1 targeting domain. A: The N-terminal region of AUP1 is represented schematically. The phospholipid bilayer is in gray, protein is in black. The targeting domain of AUP1 contains a continuous hydrophobic stretch surrounded by two hydrophilic flanks. The hydrophobic stretch of AUP1 is probably integrated into the membrane, and contains the PVG motif and the accessory motifs. The PVG motif is required for establishing monotopic/hairpin topology and LD localization. The accessory motifs (R42 and R62+R63) are required for LD targeting. B: Sequence comparison between AUP1 and the LD proteins CAV3, NSDHL, LPCAT1, and LPCAT2 (21, 32). Position 1 indicates the first residue of the hydrophobic/membrane domain. To compensate for the different length of the hydrophobic domains, we aligned the C-terminal flanks. PVG motif and similar motifs are highlighted with a black frame. Positively charged residues (R,K) in the C-terminal flank are underlined. Sequences are taken from Swissprot with the following accession numbers: AUP1 Q9Y679, CAV3 P56539, NSDHL Q15738, LPCAT1 Q8NF37, LPCAT2 Q7L5N7.