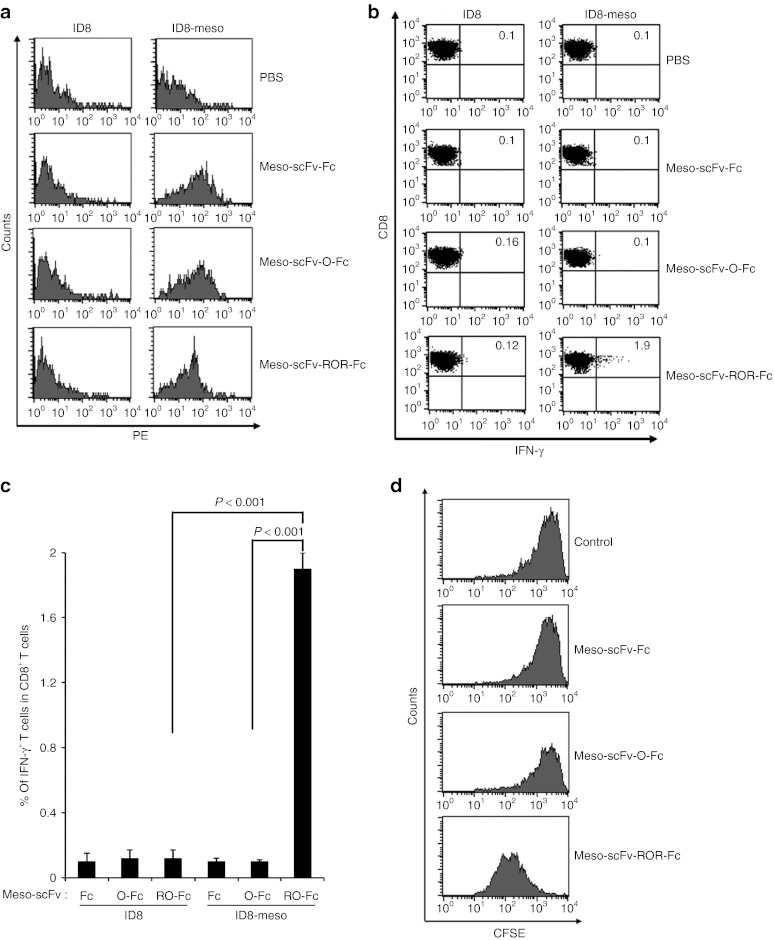
Figure 3.
Major histocompatibility complex class I presentation of ovalbumin (OVA) peptide to OVA-specific CD8+ T cells by ID8-meso cells treated with Meso-scFv-ROR-Fc in vivo. (a) Flow cytometry characterization of Meso-scFv-ROR-Fc binding to ID8-meso tumor cells. PBS was used as a negative control. Mice were i.p. injected with GFP-expressing ID8-meso or control ID8 tumor cells followed by i.p injection of different Meso-scFv-Fc chimeric proteins. Tumor cells isolated from peritoneal wash of treated tumor-bearing mice were stained with phycoerythrin (PE)-labeled antimouse Fc antibody and analyzed by flow cytometry analysis. GFP-positive cells were gated for analysis. (b) Flow cytometry characterization of OVA-specific CD8+ T-cell activation by tumor cells treated with different chimeric proteins. Tumor cells from peritoneal wash of tumor-bearing mice treated with different chimeric proteins were incubated with OVA-specific CD8+ T cells. OVA-specific CD8+ T-cell activation was determined by CD8 and intracellular interferon γ (IFN-γ) staining. (c) Representative bar graph depicting % of IFN-γ-secreting OVA-specific CD8+ T cells out of total OVA-specific T cells (mean ± SD). (d) Flow cytometry analysis to characterize the proliferation of OVA-specific CD8+ T cells in ID8-meso tumor-bearing mice treated with Meso-scFv-ROR-Fc. C57BL/six mice (five mice/group) were injected with GFP-expressing ID8-meso tumor cells (1 × 106 cells/mouse). One day later, 50 µg of different chimeric proteins and 2.5 × 106 carboxyfluorescein succinimidyl ester (CFSE)-labeled OVA-specific CD8+ T cells were i.p. injected. Three days later, washed intraperitoneal cells were stained with PE-conjugated anti-CD8 antibody then analyzed by flow cytometry to determine the extent of CFSE dilution in CFSE-labeled CD8+ T cells. Meso-scFv, mesothelin single-chain variable fragment; PBS, phosphate buffered saline.