Abstract
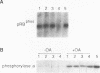
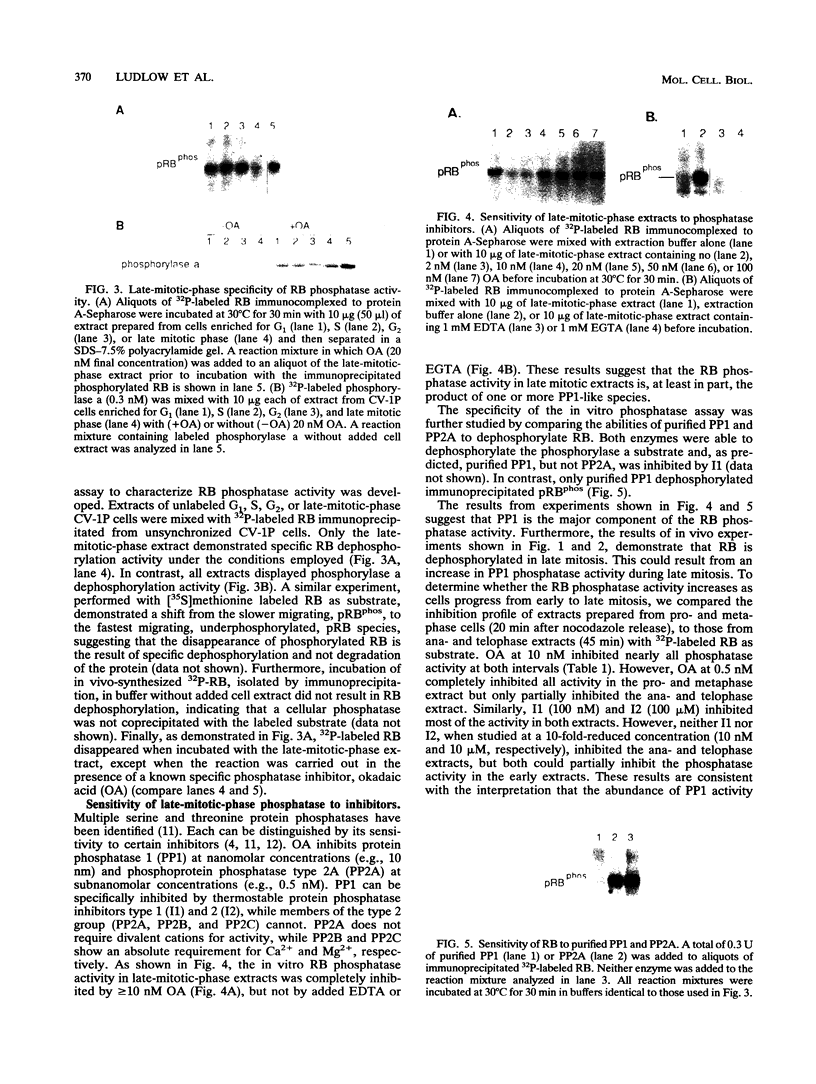
The retinoblastoma gene product (RB) undergoes cell cycle-dependent phosphorylation and dephosphorylation. Pulse-chase experiments revealed that the change in RB gel electrophoretic migration which occurs near mitosis is due to enzymatic dephosphorylation (J. W. Ludlow, J. Shon, J. M. Pipas, D. M. Livingston, and J. A. DeCaprio, Cell 60:387-396, 1990). To determine the precise timing of RB dephosphorylation and whether a specific phosphatase is active in this process, we have utilized a nocodazole block and release protocol which allows a large population of cells to progress synchronously through mitosis. In such experiments, RB dephosphorylation began during anaphase and continued until complete dephosphorylation was apparent in the ensuing G1 period. In addition, late mitotic cell extracts were capable of dephosphorylating RB in vitro. This RB-specific mitotic phosphatase activity was more active in anaphase extracts than in pro- or metaphase extracts, which is consistent with the results obtained in vivo. Okadaic acid and protein phosphatase inhibitors 1 and 2 inhibited this specific RB phosphatase activity. These results suggest a role for serine and threonine phosphoprotein phosphatase type 1 in the late mitotic dephosphorylation of RB.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axton J. M., Dombrádi V., Cohen P. T., Glover D. M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990 Oct 5;63(1):33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Shriner C. L. Methods to distinguish various types of protein phosphatase activity. Methods Enzymol. 1988;159:339–346. doi: 10.1016/0076-6879(88)59034-1. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Sunwoo J., Labbé J. C., Fernandez A., Lamb N. J. Cell cycle oscillation of phosphatase inhibitor-2 in rat fibroblasts coincident with p34cdc2 restriction. Nature. 1990 Mar 1;344(6261):74–78. doi: 10.1038/344074a0. [DOI] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Hansen M. F., Nordenskjold M., Kock E., Maumenee I., Squire J. A., Phillips R. A., Gallie B. L. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985 Apr 26;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Cohen P., Alemany S., Hemmings B. A., Resink T. J., Strålfors P., Tung H. Y. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P., Klumpp S., Schelling D. L. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989 Jul 3;250(2):596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Furukawa Y., Ajchenbaum F., Griffin J. D., Livingston D. M. The retinoblastoma-susceptibility gene product becomes phosphorylated in multiple stages during cell cycle entry and progression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1795–1798. doi: 10.1073/pnas.89.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Lamb N. J. Protein phosphatase type 1 in mammalian cell mitosis: chromosomal localization and involvement in mitotic exit. J Cell Biol. 1992 Mar;116(6):1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., DeCaprio J. A., Freedman A., Kanakura Y., Nakamura M., Ernst T. J., Livingston D. M., Griffin J. D. Expression and state of phosphorylation of the retinoblastoma susceptibility gene product in cycling and noncycling human hematopoietic cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2770–2774. doi: 10.1073/pnas.87.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Wang N. P., Qian Y. W., Lee E. Y., Lee W. H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991 Oct 18;67(2):293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Krek W., Sellers W. R., DeCaprio J. A., Ajchenbaum F., Fuchs C. S., Chittenden T., Li Y., Farnham P. J., Blanar M. A. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992 Jul 24;70(2):351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Keryer G., Borisy G. G. The mitotic spindle of Chinese hamster ovary cells isolated in taxol-containing medium. J Cell Sci. 1984 Mar;66:265–275. doi: 10.1242/jcs.66.1.265. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Buchkovich K. J., Marshak D. R., Anderson C. W., Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991 Dec;10(13):4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B. T., Gruenwald S., Morla A. O., Lee W. H., Wang J. Y. Retinoblastoma cancer suppressor gene product is a substrate of the cell cycle regulator cdc2 kinase. EMBO J. 1991 Apr;10(4):857–864. doi: 10.1002/j.1460-2075.1991.tb08018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W. Selective ability of S-phase cell extracts to dephosphorylate SV40 large T antigen in vitro. Oncogene. 1992 May;7(5):1011–1014. [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- McIntosh J. R., Koonce M. P. Mitosis. Science. 1989 Nov 3;246(4930):622–628. doi: 10.1126/science.2683078. [DOI] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew J. Y., Ling N., Yang X. M., Fodstad O., Lee W. H. Antibodies detecting abnormalities of the retinoblastoma susceptibility gene product (pp110RB) in osteosarcomas and synovial sarcomas. Oncogene Res. 1989;4(3):205–214. [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Taya Y., Yasuda H., Kamijo M., Nakaya K., Nakamura Y., Ohba Y., Nishimura S. In vitro phosphorylation of the tumor suppressor gene RB protein by mitosis-specific histone H1 kinase. Biochem Biophys Res Commun. 1989 Oct 16;164(1):580–586. doi: 10.1016/0006-291x(89)91759-2. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Borisy G. G. Anaphase onset and dephosphorylation of mitotic phosphoproteins occur concomitantly. J Cell Sci. 1989 Oct;94(Pt 2):245–258. doi: 10.1242/jcs.94.2.245. [DOI] [PubMed] [Google Scholar]
- Whitfield W. G., Gonzalez C., Maldonado-Codina G., Glover D. M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990 Aug;9(8):2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]