Abstract
Purpose
To determine the incidence of steroid induced ocular hypertension following myopic photorefractive keratectomy (PRK).
Methods
Myopic PRK was performed on 506 eyes of 269 patients. Preoperatively, spherical equivalent refractive error ranged from −1.00 to −5.00 diopters (D) and cylinder was less than 4 D. Baseline intraocular pressure (IOP) before PRK and at different time intervals after the procedure was measured by Goldmann applanation tonometry. IOP readings were corrected according to central corneal thickness as measured by Orbscan pachymetry. For the purpose of the study, corrected IOP >21 mmHg was considered as ocular hypertension.
Results
Ocular hypertension developed in 40 (7.9%) eyes overall, which occurred in 16 eyes (40%) 2–3 weeks postoperatively (mean IOP=23.5±3.0mmHg), in 20 eyes (50%) after 4–6 weeks (mean IOP=25.1±4.2 mmHg) and in 4 eyes (10%) 8–12 weeks following PRK (mean IOP=29.0±3.1 mmHg). There was no correlation between the level of IOP rise and preoperative spherical equivalent refractive error. IOP recovered to normal in all eyes after discontinuation of topical steroids and initiation of anti-glaucoma medications. Mean duration of IOP normalization was 28.5±27.7 (range 7–108 ) days and no instance of steroid-induced glaucoma was observed in any patient.
Conclusion
Topical steroids may cause ocular hypertension following PRK. Early detec-tion, prompt treatment and close follow-up are recommended. We suggest measuring IOP in post-PRK patients no later than 10 to 14 days after initiation of corticosteroid treatment.
INTRODUCTION
Photorefractive keratectomy (PRK) is generally believed to be safe and effective for treatment of myopia.1 High energy photons produced by the excimer laser machine allow controlled removal of layers of corneal tissue with micron scale precision.2 Myopic regression and corneal haze have been reported as the most common complications of PRK,3 the incidence of which tends to increase with higher attempted corrections. 4 5
Certain studies have reported that subepithelial haze resolves rapidly with topical steroids6 and steroid use has been associated with inhibition of myopic regression.6 7 One drawback is that steroid responders may develop elevated intraocular pressure (IOP) within a short time following steroid administration. Steroid induced ocular hypertension is due to reduced aqueous humor outflow facility and may occur after topical, periocular or oral glucocorticoid use.8 Elevated IOP persists as long as steroids are continued. Once steroids are withdrawn, IOP returns to baseline levels within 10 days in approximately 98% of eyes and in the rest by 3 weeks. However, there have been few cases of irreversible IOP elevation leading to glaucoma surgery.9 The risk of steroid induced ocular hypertension is higher in patients with history of open angle glaucoma, diabetes mellitus and severe myopia.8 This ocular hypertensive response to corticosteroids is an autosomal dominant trait found in one third of the general population.10 The current study was performed to evaluate the incidence of steroid induced ocular hypertension following myopic PRK.
METHODS
Data was collected by a retrospective review of patients’ records from the private practice of the senior author (MAJ). Over a three year period from January 2004 to January 2007, 629 eyes of 327 patients with simple myopia and myopic astigmatism underwent PRK. Patients were included in the study only if adequate IOP data was available. Eventually data related to 506 eyes of 269 patients with preoperative spherical refractive error of −1.00 to −5.00 diopters (D) and astigmatism less than 4 diopters was analyzed.
Photorefractive keratectomy was performed using the Nidek EC-5000 excimer laser machine (Nidek Co. Ltd., Japan). Postoperatively, a bandage contact lens (Actifresh 400, Hydron, UK) was fitted. All patients received antibiotic eye drops four times a day for 1 week and betamethasone 0.1% 4 times a day during the first 2 weeks which was substituted by fluorometholone (FML) 0.25%. The latter was tapered gradually (4, 3 and 2 times daily each for a 2 week period). Follow-up examinations were scheduled 1, 2 and 3 days, 2, 3, 4 and 6 weeks and 3, 6 and 12 months postoperatively and yearly thereafter. Intraocular pressure was measured by Goldmann applanation tonometry (GAT) at all pre- and postoperative visits. Central corneal thickness (CCT) was measured by Orbscan Π. We used a correction factor of 0.5 mmHg per 10-μm deviation from an average “normal” CCT of 540 μm for GAT readings. 11 Instances of corrected IOP higher than 21 mmHg were considered as steroid induced ocular hypertension. In any eye with corrected IOP more than 21 mmHg, treatment with timolol 0.5% was started and dorzolamide 2% was added if the pressure was still uncontrolled. Closer follow-up examinations (every 1 to 2 weeks) were scheduled in patients who developed ocular hypertension.
RESULTS
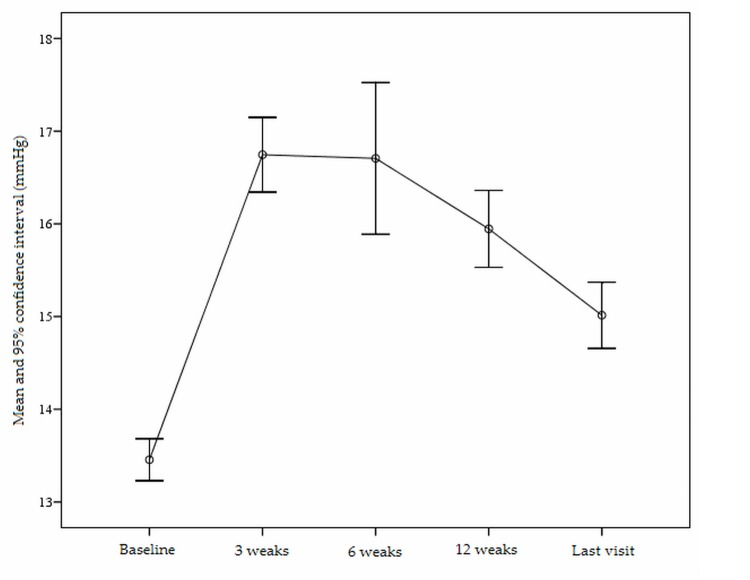
Preoperative patient data are presented in table 1. Patients’ age ranged from 18 to 25 (mean 26±6) years and 68.8% of the cases were female. Median follow-up was 9.5 (range 3–62) months. Overall, steroid induced ocular hypertension occurred in 40 eyes (7.9%) including 16 eyes (40%) in which IOP rise (mean IOP= 23.5±3.0 mmHg) occurred 2–3 weeks postoperatively, 20 eyes (50%) in which IOP elevation (mean IOP= 25.1±4.2 mmHg) was observed 4–6 weeks after PRK and 4 eyes (10%) in which IOP rise (mean IOP= 29.0±3.1 mmHg) developed 8–12 weeks postoperatively. No significant correlation was observed between IOP rise and preoperative spherical equivalent refractive error. IOP returned to normal levels in all eyes after discontinuation of topical steroids and use of glaucoma medications. Mean duration of IOP normalization was 28.5±27.7 (range 7–108) days. Mean postoperative corrected IOP was higher than preoperative values at different time intervals. Mean corrected IOP was 16.8±3.1 mmHg (3.6±3.2 mmHg rise, P<0.001) at 3 weeks; 16.7±4.8 mmHg (3.1±4.4 mmHg elevation, P<0.001) at 6 weeks; 15.9±3.0 mmHg (2.5±3.0 mmHg increase, P<0.001) at 12 weeks; and 15.0±2.7 mmHg (1.6±2.9 mmHg higher than preoperative IOP, P<0.001) at final examination (Fig. 1). According to visual field testing, none of the patients developed steroid induced glaucoma in any eye.
Table 1.
Preoperative patient data
| Age (years) | |
| M±SD | 26±6 |
| Range | 18-45 |
| Sex | |
| Male | 84 (31.2%) |
| Female | 185 (68.8%) |
| Spherical equivalent (D) | |
| M±SD | -3.09±0.9 |
| Range | −1.0 to −5.0 |
| Corrected IOP (mmHg, M±SD) | |
| Preoperative | 13.44±2.57 |
| Preoperative | 15.01±2.74 |
M, mean; SD, standard deviation; D, diopter; IOP, intraocular pressure
Figure 1.
Changes of intraocular pressure at different time intervals during the study period.
DISCUSSION
Topical steroids are used at different time points in post-PRK patients: in the early postoperative period to suppress inflammation, 3 to 4 months after the procedure to modulate wound healing, and later to treat regression. There is little controversy regarding the early postoperative administration of topical steroids, but there is no consensus on the prolonged use of topical steroids in the routine post-PRK patient.
McLean12 was the first to report the IOP elevating effect of the adrenocorticotropic hormone and cortisone. Steroid induced glaucoma was well characterized by Armaly13 in the 1960s. These investigators reported that after receiving dexamethasone or betamethasone 3 to 4 times daily for 4 weeks, 34% to 42% of normal individuals demonstrate an IOP rise from a baseline value of 6 to 15 mmHg to a final level of 20 to 31 mmHg.
Steroid induced ocular hypertension and glaucoma are believed to result from increased aqueous outflow resistance. Based on histologic studies of human trabecular meshwork (TM) specimens from eyes with steroid induced glaucoma and experimental studies on organcultured human eyes and cultured TM cells, several mechanisms for steroid-induced IOP elevation have been proposed, which include: accumulation or deposition of extracellular matrix material, decreased protease and stromelysin activity, reorganization of the TM cytoskeleton, increased nuclear size and DNA content, decreased phagocytotic capacity, and changes in the synthesis of specific proteins.14 Increased laminin deposition is also reported to be responsible for decreased outflow facility both in steroid induced glaucoma and in primary open-angle glaucoma.15 Moreover, it has been demonstrated that the expression of the myocilin protein (previously known as TIGR and GLC1A) is greatly enhanced by glucocorticoids in cultured TM cells. The MYOC (myocilin) gene has been closely linked to open-angle glaucoma.14
According to Seiler et al5 and Machat et al16 steroid induced IOP elevation is a prevalent, early postoperative complication of PRK, occurring in 8 to 32% of treated eyes. Shimizu et al17 reported the incidence of post-PRK IOP rise (>21mmHg) to be 8.9%. Gartry et al18 reported a post-PRK steroid response of 12% in their series. In our study, steroid induced ocular hypertension occurred in 7.9% of eyes. Most of our patients developed ocular hypertension 4 to 6 weeks after PRK, which is similar to Nagy's study.7 In the current series none of the cases developed glaucomatous damage.
One limitation of our study is that IOP measurement was performed by GAT. Despite the introduction of various correction factors, the validity of IOP readings by Goldmann type applanation tonometers after keratorefractive procedures has been questioned.19 Relative flattening and thinning of the central cornea following excimer laser treatment may contribute to a falsely low IOP reading by GAT.20 21 Ehlers et al20 found that the discrepancy between actual IOP and IOP readings by GAT was linearly correlated with CCT. Under- and overestimate of IOP measurement may occur with thinner or thicker corneas, respectively, with an average error of 0.7 mmHg per 10 μm deviation from a "normal" CCT value of 520 μm considered in the manufacture of the GAT. Kohlhaas et al22 employed a closed, manometrically controlled system to determine the effect of CCT, corneal curvature and axial length on applanation tonometry in patients scheduled for cataract surgery. They reported an error of 0.4 mmHg in IOP measured by applanation tonometry compared to actual IOP per 10 μm change in CCT. They found that IOP measurements by applanation tonometry were linearly correlated with CCT at different IOP levels, but no correlation existed between IOP and corneal curvature or axial length. Pascal dynamic contour tonometry (DCT) is a device designed to measure IOP independent of corneal thickness and curvature, and ocular rigidity. There are numerous reports that DCT may be more accurate in IOP measurement in eyes with thin corneas or following refractive laser surgery.23
Several studies have investigated the effect of PRK on IOP measurement with the Goldmann tonometer. Schipper et al24 found a 2 to 3 mmHg decrease in IOP after PRK, Faucher et al25 reported a mean drop of 2.4 mmHg, Chatterjee et al19 observed a 3.1 mmHg decrease, but in contrast, Kohlhaas et al26 noted a 0.1 mmHg increase. We found that postoperative corrected IOP had an increasing pattern as compared to preoperative values (Fig. 1); 6 weeks postoperatively, mean corrected IOP was 16.7±4.8 mmHg which was 3.1±4.4 mmHg higher than mean preoperative IOP. After that period, corrected IOP decreased, but never reached preoperative levels.
Using a non-contact tonometer, Chatterjee et al19 found significant correlation between the change in IOP and preoperative spherical equivalent refractive error which is in contrast to the work by Faucher et al.25 We also found no significant correlation between IOP increment and depth of ablation.
In summary, topical steroids seem to cause secondary ocular hypertension in a significant minority of patients following PRK which does not seem to be correlated with the magnitude of refractive error or depth of ablation. Early detection of IOP rise and timely treatment with pressure lowering agents and close follow-up are recommended. We suggest measuring IOP in post-PRK patients no later than 2 weeks after initiation of corticosteroid treatment. It may be prudent to substitute potent steroids such as betamethasone with weaker agents with less propensity for IOP elevation such as fluorometholone.
REFERENCES
- 1.Goggin MJ, Kenna PF, Lavery FL. Photoastigmatic refractive keratectomy for compound myopic astigmatism with a Nidek laser. J Refract Surg. 1997;13:162–166. doi: 10.3928/1081-597X-19970301-13. [DOI] [PubMed] [Google Scholar]
- 2.Trokel S. Evolution of excimer laser cornealsurgery. J Cataract Refract Surg. 1989;15:373–383. doi: 10.1016/s0886-3350(89)80054-9. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Ophthalmology. Excimer laser photorefractive keratectomy (PRK) for myopia and astigmatism. Ophthalmology. 1999;106:422–437. [PubMed] [Google Scholar]
- 4.Kremer I, Kaplan A, Novikov I, Blumenthal M. Patterns of late corneal scarring after photorefractive keratectomy in high and sever myopia. Ophthalmology. 1999;106:467–473. doi: 10.1016/S0161-6420(99)90104-5. [DOI] [PubMed] [Google Scholar]
- 5.Seiler T, Holschbach A, Derse M, Jean B, Genth U. Complications of myopic photorefractive keratectomy with excimer laser. Ophthalmology. 1994;101:153–160. doi: 10.1016/s0161-6420(94)31371-6. [DOI] [PubMed] [Google Scholar]
- 6.Tengroth B, Epstein D, Fagerholm P, Hamberg-Nystorm H, Fitzsimmons TD. Excimer laser Photorefractive keratectomy for myopia; clinical results in sighted eyes. Ophthalmology. 1993;100:739–745. doi: 10.1016/s0161-6420(93)31581-2. [DOI] [PubMed] [Google Scholar]
- 7.Nagy ZZ, Szabo A, Kruger RR, Suveges I. Treatment of intraocular pressure elevation after photorefractive keratectomy. J Cataract Refract Surg. 2001;27:1018–1024. doi: 10.1016/s0886-3350(01)00889-6. [DOI] [PubMed] [Google Scholar]
- 8.Vetrugno M, Maino A, Quaranta M, Cardia L. A randomized, comparative open-label study on the efficacy of latanaprost and timolol in steroid induced ocular hypertension after photorefractive keratectomy. Eur J Ophthalmol. 2000;10:205–211. doi: 10.1177/112067210001000303. [DOI] [PubMed] [Google Scholar]
- 9.Munjal VP, Dhir SP, Jain IS. Steroid induced glaucoma. Indian J Ophthalmol. 1982;30:379–382. [PubMed] [Google Scholar]
- 10.Havener WH. Ocular PharmacologyOcular Pharmacology. 4th ed. USA: Mosby; 1978. Corticosteroid therapy; pp. 347–405. [Google Scholar]
- 11.Zarab RA. American Academy of Ophthalmology. Basic and clinical science course: Glaucoma. The Academy; 2004-2005. Intraocular pressure and aqueous humor dynamics; pp. 40–45. [Google Scholar]
- 12.McLean JM, Woods AC. Clinical and experimental observation on the use of ACTH and cortisone in ocular inflammatory disease. Trans Am Ophthalmol Soc. 1950;48:293–294. [PMC free article] [PubMed] [Google Scholar]
- 13.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. I: the effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi T, Takagi Y, Mori K, Nishino H, Yue BYLT, Kinoshita S. cDNA microarray analysis of gene expression changes induced by dexamethasone in cultured human trabecular meshwork cells. Invest Ophthalmol. 2002;43:3691–3697. [PubMed] [Google Scholar]
- 15.Dickerson JE, Steely HT, Clark AF. The effect of dexamethasone in integrin and laminin expression in cultured human trabecular meshwork cells. EXP Eye Res. 1998;66:731–738. doi: 10.1006/exer.1997.0470. [DOI] [PubMed] [Google Scholar]
- 16.Machat JJ, Tayfour F. Photorefractive keratectomy for myopia: preliminary results in 147 eyes. Refract Corneal Surg. 1993;9(suppl):S16–S19. [PubMed] [Google Scholar]
- 17.Shimizu K, Amano S, Tanaka S. Photorefractive keratectomy for myopia: One-year follow-up in 97 eyes. Refract Corneal Surg. 1994;10(Suppl):S178–187. [PubMed] [Google Scholar]
- 18.Gartry DS, Kerr Muir MG, Marshall J. Excimer laser photorefractive keratectomy.18-month follow-up. Ophthalmology. 1992;99:1209–1219. doi: 10.1016/s0161-6420(92)31821-4. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee A, Shah S, Bessant DA, Naroo SA, Doyel SJ. Reduction in intraocular pressure after Excimer laser photorefractive keratectomy; correlation with pretreatment myopia. Ophthalmology. 1997;104:355–359. doi: 10.1016/s0161-6420(97)30308-x. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson M, Kass MA, Moses RA, Grodzki WJ. Increased corneal thickness simulating elevated intraocular pressure. Arch Ophthalmol. 1978;96:664–665. doi: 10.1001/archopht.1978.03910050360012. [DOI] [PubMed] [Google Scholar]
- 22.Kohlhass M, Bohem AG, Spoerl E, Pursten A, Grein H, Pilluant LE. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol. 2006;25:471–476. doi: 10.1001/archopht.124.4.471. [DOI] [PubMed] [Google Scholar]
- 23.Ozbek Z, Cohen EJ, Hammersmith KM, Rapuano CJ. Dynamic contour tonometry: a new way to assess intraocular pressure in ectatic corneas. Cornea. 2006;25:890–894. doi: 10.1097/01.ico.0000224649.12214.33. [DOI] [PubMed] [Google Scholar]
- 24.Schipper I, Senn P, Thomann U, Suppiger M. Intraocular pressure after excimer laser photorefractive keratectomy for myopia. J Refract Surg. 1995;11:366–370. doi: 10.3928/1081-597X-19950901-13. [DOI] [PubMed] [Google Scholar]
- 25.Faucher A, Gregoire J, Blondeau P. Accuracy of Goldmann tonometry after refractive surgery. J Cataract Refract Surg. 1997;23:832–838. doi: 10.1016/s0886-3350(97)80239-8. [DOI] [PubMed] [Google Scholar]
- 26.Kohlhaas M, Lerche RC, Draeger J, Armott E. The influence of corneal thickness and corneal curvature on tonometry readings after corneal refractive surgery. Eur J Implant Refract Surg. 1995;7:84–88. [Google Scholar]