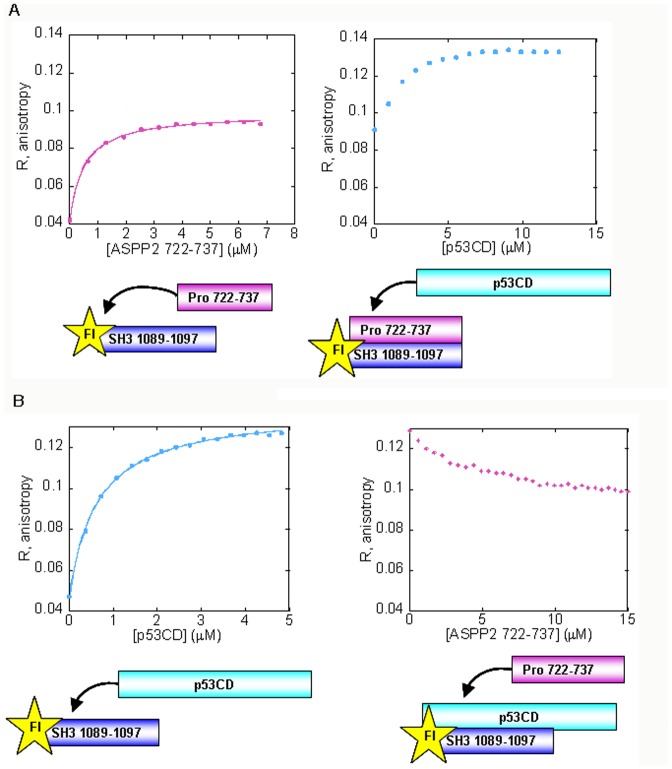
Figure 2. p53CD and ASPP2 Pro 723–737 compete for binding the Fl-ASPP2 n-src loop peptide residues 1089–1097: fluorescence anisotropy experiments.
(A) A peptide derived from the proline rich domain of ASPP2 residues 722–737 binds the ASPP2 n-src peptide with K d = 0.50±0.03 µM (magenta); Following titration of p53CD (cyan) into to the fully formed complex between Fl-ASPP2 1089–1097 and ASPP2 722–737, the anisotropy increased (B) p53CD residues 94–312 bind the ASPP2 n-src peptide with K d = 0.63±0.02 µM (cyan). Unlabeled ASPP2 722–737 (magenta) displaced p53CD form its fully formed complex with ASPP2 1089–1097.