Figure 7.
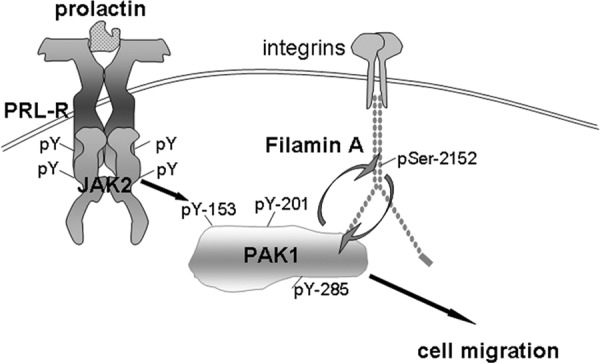
Schematic representation of the proposed working model. Initiation of PRL signaling involves PRL binding to PRL receptor (PRL-R) at the plasma membrane and activation of the tyrosine kinase JAK2, which, in turn, phosphorylates PRL-R. Phosphorylated tyrosines within the receptor and JAK2 recruit an array of signaling proteins, including PAK1. JAK2 tyrosyl phosphorylates PAK1 on Tyr(s) 153, 201, and 285, thereby increases PAK1 activities (both the serine/threonine kinase activity and ability to create potential protein-protein interactions) and stimulates phosphorylation of FLNa on Ser 2152. Phosphorylated FLNa has increased actin-regulating activity to stimulate cell migration and enhances PAK1 kinase activity by a positive feedback loop.
