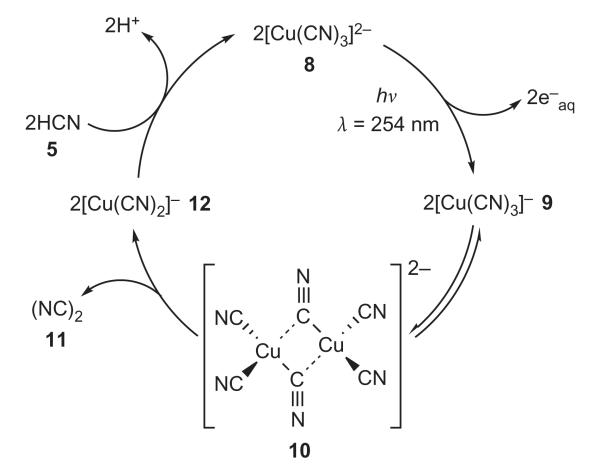
Figure 2. Photoredox cycling of copper cyanide complexes in the presence of hydrogen cyanide 5.
Each turn of the cycle results in the oxidation of two molecules of hydrogen cyanide 5 to cyanogen 11, with the concomitant production of two protons and two hydrated electrons. The photoxidation of the tricyanocuprate(I) species 8 to tricyanocuprate(II) 9 is inferred because the corresponding dicyanocuprate(I) is not photoactive at 254 nm, and, according to stability constant calculations, the tetracyanocuprate(I) is a very minor species33. Dimerization of 9 to the binuclear complex 10 and reductive elimination of cyanogen 11 giving dicyanocuprate(I) 12 is suggested by the kinetics of the decomposition of copper(II) cyanide complexes34. Finally, conversion of dicyanocuprate(I) 12 to tricyanocuprate(I) 8 is suggested by the aforementioned photoinactivity of 12 and stability constant data, and the fact that a cycle is clearly operative.