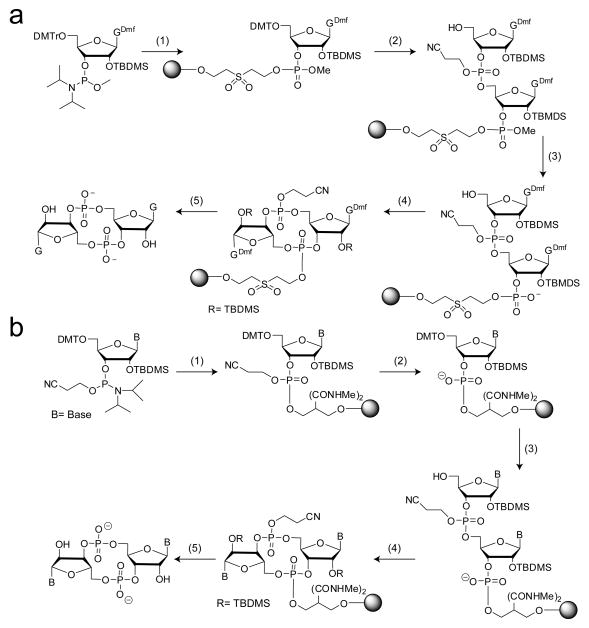
Figure 6.
Solid phase methods for c-di-GMP synthesis utilizing on-bead cyclization procedures. (a) Method developed by Kiburu et al93. Two different phosphoramidites containing different phosphate protecting groups were used. 1) i) solid support, tetrazole/ACN ii) I2, pyridine, H2O; 2) i) DCA/DCM ii) cyanoethyl phosphoramidite, tetrazole/ACN iii) I2 pyridine, H2O iv) DCA/DCM; 3) S2Na2 4) MSNT, pyridine 5) i) aqueous NH3 ii) HF-TEA. For the solution phase cyclization method, O-methyl phosphoramidtes were used in both coupling steps and the linear dinucleotide was cleaved from the bead prior to cyclization and global deprotection. (b) Modified solid-phase synthesis of c-di-GMP, base and ribose modified analogs. Cyanoethyl-protected phosphoramidites were used for both coupling reactions. 1) i) tetrazole/ACN ii) tBuOOH iii) acetic anhydride/methylamine; 2) i) 50% TEA/ACN, 2 hours; 3) i) 3% DCA/DCM ii) tetrazole/ACN + CNE phosphoramidite iii) tBuOOH iv) acetic anhydride/methylamine 4) 0.1M MSNT, 72–96 hours; 5) i) ammonium hydroxide ii) HF-TEA (for 2′-OH analogs only).