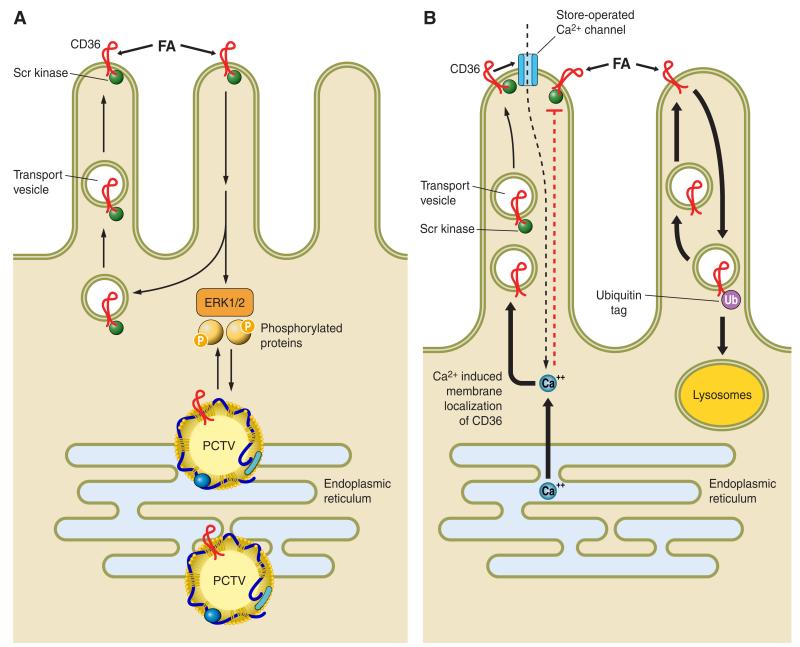
FIGURE 1.
Aspects of CD36 signaling and trafficking that may have potential relevance to the regulation of intestinal lipid metabolism. The figure proposes some hypothetical working concepts regarding steps that may be involved in the effect of intestinal CD36 to facilitate fatty acid (FA) uptake and chylomicron formation. A: CD36 is highly expressed on enterocytes of the proximal intestine and localizes to the apical side of intestinal villi (132, 155, 170) where it interacts with FA released from the digestion of dietary triglycerides. While CD36 functions in facilitating uptake of FA by proximal enterocytes (155, 183), it does not contribute quantitatively to FA absorption (47, 156), since it would be rapidly saturated at the levels of monomeric FA present in the lumen. However, CD36-mediated FA uptake and associated signaling, operating at the very early stages of absorption, may promote intracellular events that facilitate chylomicron assembly (46, 88, 156). CD36 signaling is in most cases initiated via the CD36-associated Src kinases and downstream via the extracellular regulated kinase ERK1/2 (109). This signaling pathway may be important for phosphorylating proteins required for coordinating ER processing of prechylomicron vesicles (PCTV). CD36 has also been identified in the protein complex that is required for formation of the PCTV (190). B: recent data indicate that CD36 signaling may also be mediated by a rise in intracellular calcium. Calcium release from the ER promotes membrane CD36 localization, which regulates calcium influx via the store-operated calcium channel. The sustained increase in intracellular calcium could influence multiple events related to lipid processing or secretion (16, 109). B also illustrates the concept that CD36 is downregulated by FA via ubiquitination and lysosomal degradation (196), and this feedback loop may work to reduce CD36 function in the presence of high concentrations of long-chain fatty acids (LCFA) (207). In the enterocytes, the LCFA-induced decrease in CD36 associates with reduced activation of ERK1/2 which may serve to upregulate MTTP abundance (138).