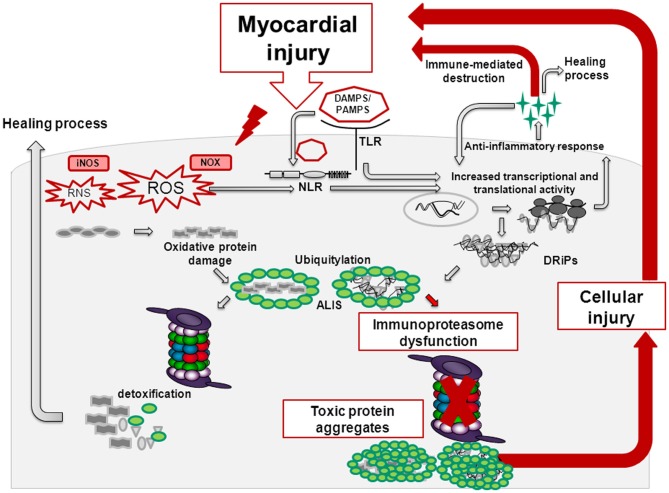
Figure 1.
Myocardial injury induces reactive oxygen species (ROS) and reactive nitrogen species (RNS) via the activity of NADPH-oxidase (NOX) and nitric oxide synthase (NOS). Thereby, endogenous material being referred to as danger-associated molecular patterns (DAMPs) is released. DAMPs activate membrane-bound Toll-like receptors (TLRs) and cytoplasmatic Nucleotide-binding/oligomerization domain-like receptors (NLRs). TLR and NLR-signaling results in cytokine and chemokine release. Chemokines attract inflammatory cells and thereby facilitate tissue repair. Upon activation of the mammalian target of rapamycin (mTOR)-pathway, cytokines activate the cellular translation machinery giving rise to an increased pool of misfolded, damaged proteins known as defective ribosomal products (DRIPs). These nascent proteins are prone to be further modified by ROS/RNS eventually leading to an imbalance in the protein homeostasis with a surplus of misfolded, oxidant-damaged proteins in the cell. Here, regulatory components within the proteasomes take action—in inflammation, immunoproteasomes with increased proteolytic activity are formed that ensure the timely degradation of these inherently toxic protein aggregates. However, whenever the immunoproteasome is dysfunctional or not properly assembled, proteotoxic aggregates accumulate thereby promoting cell death. This in turn creates a vicious cycle eventually potentiating cardiac remodeling and heart failure.