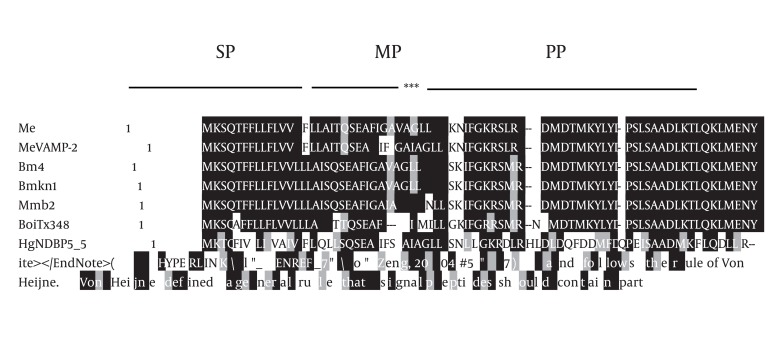
Figure 2. Multiple Sequence Alignment of Meamp Toxin-Like Peptide (Me) and Other Scorpion Toxins.
The amino acid sequence of MeAMP toxin-like peptide was aligned with venom antimicrobial peptide-2 (MeVAMP-2) (ABR20120) from species of lesser Asian scorpion M. eupeus, biologically active peptide 4 from Mesobuthus martensii (AF151795), venom peptide precursor (BmKn1) from Buthus martensii (AF150010), antimicrobial protein b2 (Mmb2) from Mesobuthus martensii, putative toxin Tx348 (BoiTx348) (FJ360839) from Buthus occitanus israelis, linear non-disulfide bridged peptide 5.5 (HgNDBP5_5) (FM998743) from Hadrurus gertschi, partial antimicrobial peptide NDPB 5.7 precursor (OcNDPB5_7) (FM998743) from Opisthacanthus cayaporum and cytotoxic linear peptide IsCT1 precoursor (OmIsCT1) (AF397895) from Opisthacanthus madagascariensis. The amino acids are denoted by one-letter symbols. Shading indicates identity (black) or conservative substitutions (grey) relative to MeAMP toxin-like peptide. Gaps represented by dashes were introduced to maximize the alignment. The cDNA encodes a signal peptide (SP), mature peptide (MP), and a propeptide (PP) were indicated. The Gly–Lye–Arg pattern that is required for posttranslational processing and the C-terminal amidation of the mature peptide is indicated by stars.