Figure 2. The Extended Inserts of Ath α-DOX.
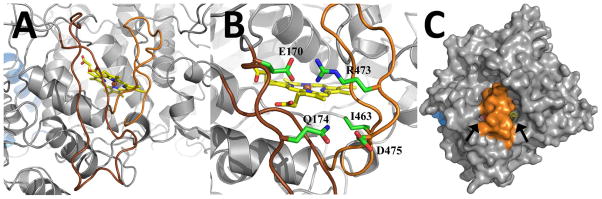
(A) View of the two extended inserts that lie at the surface of Ath α-DOX and cover the heme. The 170–197 loop is colored in brown and the 461–478 loop is colored in orange. (B) View of the ionic interactions between the two inserts that serve to stabilize and rigidify the loops. The side chains of Glu-170, Gln-174, Ile-463, Arg-473, and Asp-475 are labeled accordingly. (C) Surface representation of Ath α-DOX that depicts the location of the two small channels (black arrows) that provide access to the heme. The base and catalytic domain are colored in blue and grey, respectively, while residues 170–197 and 461–478 are colored in orange.
