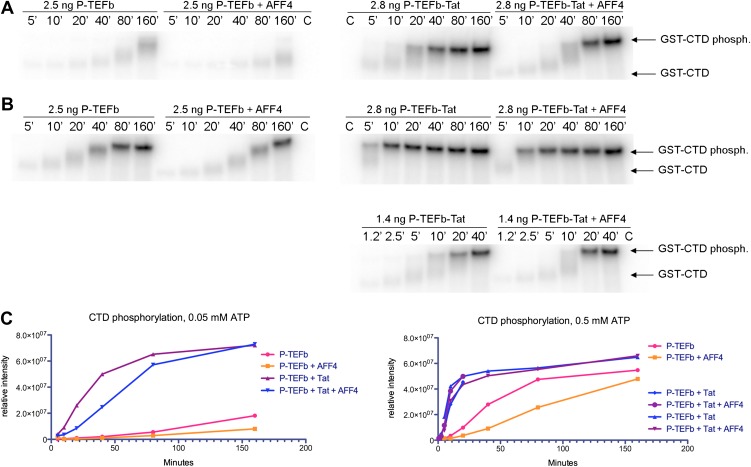
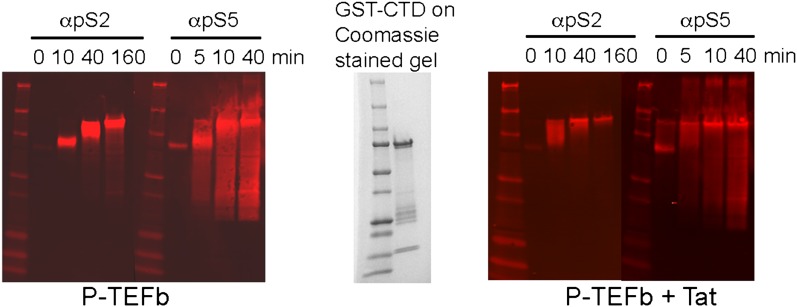
Figure 5. Kinase activity of P-TEFb and P-TEFb-Tat complexes with AFF4.
(A) Autoradiogram showing phosphorylation of GST-CTD (500 ng) by P-TEFb and P-TEFb-Tat with and without excess (0.28 μM) AFF42-73 in the presence of low (50 μM) ATP. (B) Phosphorylation of 500 ng GST-CTD by P-TEFb and P-TEFb-Tat with and without excess (0.28 μM) AFF42–73 in the presence of saturating (500 μM) ATP. AFF4 reduces the activity of P-TEFb twofold and has little influence on the kinase activity of Tat-P-TEFb. Tat-P-TEFb is, however, sevenfold to tenfold more active than P-TEFb. Lane 3 in panels (A and B) is a control without GST-CTD. (C) Quantitation of the radioactive GST-CTD in (A and B).
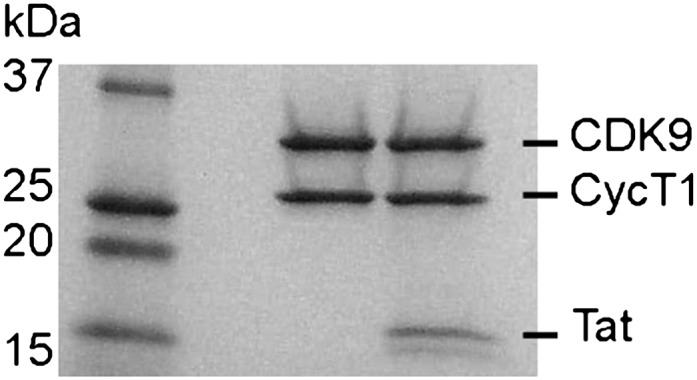
Figure 5—figure supplement 1. SDS polyacrylamide gel of P-TEFb and P-TEFb-Tat at the same ratio as they were used in the kinase assay.