Abstract
Objective:
To measure the volume of basal forebrain septal nuclei in patients with temporal lobe epilepsy (TLE) as compared to patients with extratemporal epilepsy and controls. In animal models of TLE, septal lesions facilitate epileptogenesis, while septal stimulation is antiepileptic.
Method:
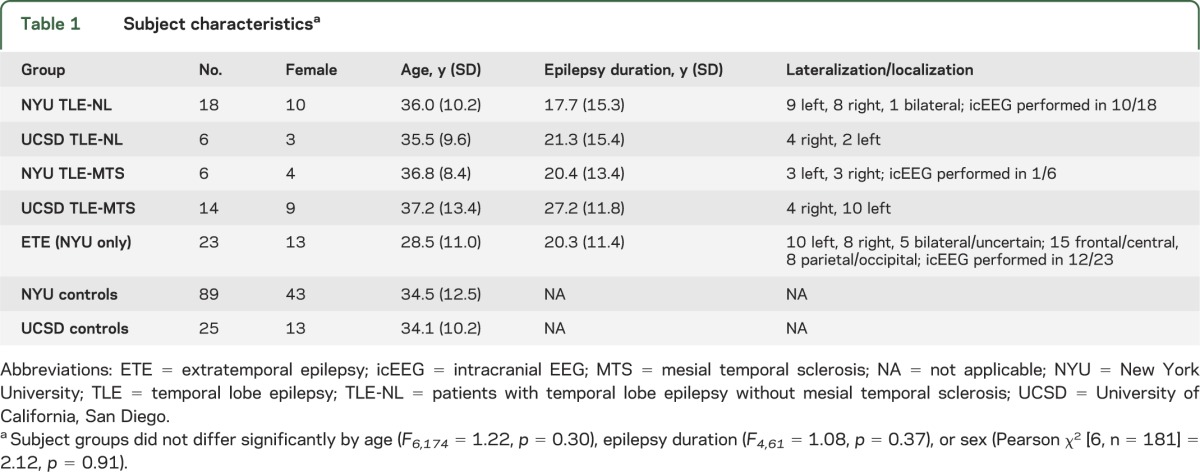
Subjects were recruited from 2 sites and consisted of patients with pharmacoresistant focal epilepsy (20 with TLE and mesial temporal sclerosis [MTS], 24 with TLE without MTS, 23 with extratemporal epilepsy) and 114 controls. Septal volume was measured using high-resolution MRI in association with newly developed probabilistic septal nuclei maps. Septal volume was compared between subject groups while controlling for relevant factors.
Results:
Patients with TLE without MTS had significantly larger septal nuclei than patients with extratemporal epilepsy and controls. This was not true for patients with MTS. These results are interpreted with reference to prior studies demonstrating expansion of the septo-hippocampal cholinergic system in animal models of TLE and human TLE surgical specimens.
Conclusion:
Septal nuclei are enlarged in patients with TLE without MTS. Further investigation of septal nuclei and antiepileptic septo-hippocampal neurocircuitry could be relevant to development of new therapeutic interventions such as septal stimulation for refractory TLE.
Human septal nuclei, located in the basal forebrain, consist of the medial septum and diagonal band of Broca. Septal nuclei provide the main cholinergic input to the hippocampus via the fimbria/fornix1 and are critical for hippocampal theta oscillations that mediate learning and memory.2,3 In animal models of temporal lobe epilepsy (TLE), medial septal lesions facilitate epileptogenesis, and chemical or electrical septal stimulation can stop and prevent seizures.4–8 To our knowledge, no studies have examined septal nuclei structure in human TLE.
We used MRI in association with probabilistic maps of human septal nuclei9 to measure septal volume in patients with TLE either with or without mesial temporal sclerosis (MTS), patients with partial epilepsy not involving the hippocampus (extratemporal epilepsy [ETE]), and healthy controls. Based on prior human MRI studies showing atrophy of hippocampi and connected limbic regions in TLE including the fornix,10,11 we initially hypothesized that septal volume would be reduced in TLE. Contrary to this hypothesis, we found septal enlargement in TLE without MTS.
METHODS
Subjects
All subjects provided informed consent to participate in this institutional review board–approved study. Patients with epilepsy were recruited from either the New York University (NYU) or University of California, San Diego (UCSD) Epilepsy Center. See table 1 for subject information. All patients had medically intractable seizures and were being considered for epilepsy surgery. Seizure focus lateralization/localization was made by experienced epileptologists based on clinical criteria including neuroimaging, video-EEG, and intracranial EEG (icEEG) when available. Patients with TLE were categorized as having MTS (TLE-MTS) or not based on standard criteria.12 Patients without MTS are referred to as TLE-NL, though 5/24 did have subtle mesial temporal abnormalities that did not qualify as MTS (1 with subtle parahippocampal gray–white blurring, 1 with slight hippocampal increased fluid-attenuated inversion recovery signal but no atrophy, 3 with mild atrophy but no signal change). Patients with TLE due to tumors or other non-MTS lesions were not included. Patients with ETE had normal MRI (n = 15), incidental noncortical abnormalities (n = 2), or subtle focal cortical dysplasia (FCD) characterized by signal change or gray–white blurring at the seizure focus (n = 4). Patients with grossly abnormal MRI which could interfere with image processing, serious medical/neurologic disease other than epilepsy, or serious psychiatric disease such as psychosis were not included. Controls were recruited through online advertisement and denied medical, neurologic, or psychiatric illness.
Table 1.
Subject characteristicsa
MRI scanning
High-resolution research MRI was performed at either the NYU Center for Brain Imaging on a 3 T Siemens (Munich, Germany) Allegra head-only scanner or at the UCSD Radiology Imaging Laboratory on a GE (Schenectady, NY) 1.5 T EXCITE scanner (NYU: echo time [TE] = 3.25 ms, repetition time [TR] = 2,530 ms, inversion time [TI] = 1.1 ms, field of view [FOV] = 256 mm, voxel size = 1 × 1 × 1.33 mm; UCSD: TE = 3.8 ms, TR = 10.7 ms, TI = 1,000 ms, FOV = 240 mm, voxel size = 1.25 × 1.25 × 1.2 mm).
Measurement of septal volume
Using statistical parametric mapping (SPM8, Wellcome Trust Center for Neuroimaging, London, UK), individual scans were normalized to the Montreal Neurological Institute (MNI) 152 T1-template using a 12-parameter affine transformation and partitioned into gray and white matter using a unified segmentation approach. Gray and white matter maps were registered to the segmented MNI 152 T1 template using the DARTEL toolbox, an efficient large deformation diffeomorphic framework which provides information about voxel-level local expansion and contraction necessary to deform the image to match the template.13 The DARTEL flow fields derived from this registration were applied to a binary mask of the septal nuclei (generated as described below). To warp the septal maps, which were in MNI template space, back to each individual subject's native space, we applied the inverse DARTEL flow field. We then calculated the volume of each subject's septal mask in mm3. We have used this method previously.14
The binary septal nuclei masks we used were based on probabilistic maps of magnocellular (presumably cholinergic) basal forebrain cell groups generated using digital images of cell-stained histologic sections from 10 human postmortem brains that were reconstructed in 3D using the MRI scans of the fixed brain as a shape reference, then spatially normalized to the single-subject T1-weighted MNI reference brain, as described in detail elsewhere.9 The masks included all voxels showing ≥10% probability of being part of the medial septal nucleus or the nucleus of the diagonal band of Broca, corresponding to Mesulem's Ch1-Ch2 cell group.1,9
To allow correction for baseline differences in intracranial volume across subjects, total intracranial volume (TICV) was calculated using Freesurfer 5.0 (http://surfer.nmr.mgh.harvard.edu).
Statistical analysis
Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL). NYU and UCSD datasets were combined and were also analyzed separately. One-way analysis of covariance was conducted with independent variable = subject group (TLE, ETE [for NYU dataset only], and controls); covariates = age, TICV, and, when appropriate, site; and dependent variable = total septal volume. To assess an effect of MTS on septal volume, analyses were also performed with patients with TLE split into separate groups based on the presence or absence of MTS (TLE-MTS, TLE-NL). For patient groups, partial correlation analyses were performed to assess a possible effect of epilepsy duration on septal volume while controlling for age, TICV, and, when appropriate, site.
Because septal nuclei are located adjacent to and in between lateral ventricles, and ventricular enlargement (which can occur in TLE15) could potentially distort septal volume measurement, we used Freesurfer to calculate total lateral ventricle volume (TLVV), and performed all analyses both with and without TLVV as an additional covariate.
All results were considered significant at p < 0.05.
RESULTS
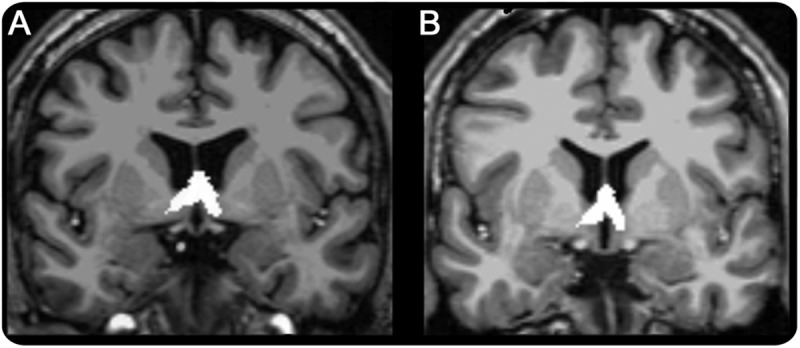
Mean septal volumes are presented in table 2. The figure shows an example of septal nuclei segmentation.
Table 2.
Mean septal volumes
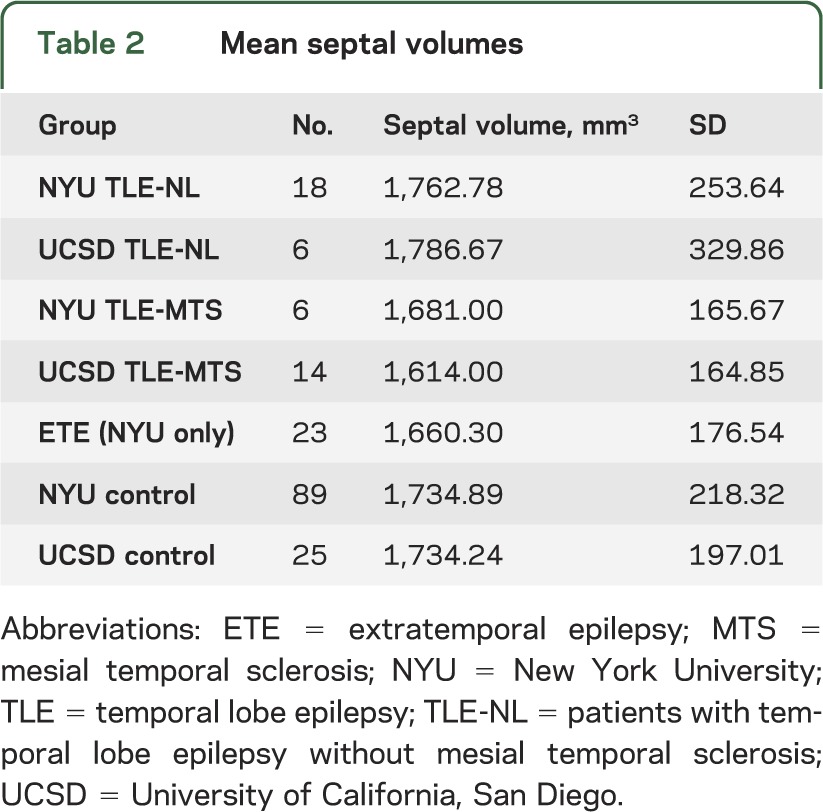
Figure. Coronal view of septal nuclei masks in (A) a patient with temporal lobe epilepsy and (B) a control.
TLE vs controls
There was a highly significant effect of group, indicating that patients with TLE had larger septal nuclei than controls (F1,153 = 12.9, p < 0.0005) when controlling for age, TICV, and site. These results were similar when TLVV was included as an additional covariate (p = 0.002), and when NYU and UCSD datasets were analyzed separately (p = 0.017 and p = 0.037, respectively).
TLE-NL vs TLE-MTS vs controls
When the TLE group was divided into those with and without MTS, septal volume still differed significantly by group (F2,152 = 7.432, p = 0.001), and pairwise comparisons indicated that only TLE-NL patients had significantly larger septal volumes than controls (p < 0.0005). TLE-MTS patients did not differ from TLE-NL patients (p = 0.172) or from controls (p = 0.120). Results when TLVV was included as an additional covariate were similar (p < 0.0005), except a trend for greater septal volume in TLE-NL as compared to TLE-MTS patients became apparent (p = 0.079). Note that for this comparison, NYU and UCSD datasets were not analyzed separately because there were only 6 NYU TLE-MTS patients and only 6 UCSD TLE-NL patients.
TLE-NL vs TLE-MTS vs ETE vs controls
In the NYU dataset, which included patients with ETE, septal volume differed significantly by group (F3,130 = 3.808, p = 0.012), and pairwise comparisons indicated that TLE-NL patients had significantly larger septal volumes than controls (p = 0.002) and patients with ETE (p = 0.004). There was a weak trend for TLE-NL patients to have larger septal volume than TLE-MTS patients (p = 0.093). TLE-MTS patients did not differ from controls (p = 0.910) or from patients with ETE (p = 0.778). Results were similar when TLVV was included as an additional covariate in analysis (p < 0.0005).
Correlation with epilepsy duration
There was no correlation between septal volume and epilepsy duration when controlling for age, TICV, and site in patients with TLE overall (r = 0.088, p = 0.584) or in TLE-MTS or TLE-NL subgroups (r = 0.142, p = 0.587; r = 0.111, p = 0.632, respectively). These results did not change when TLVV was included as an additional covariate, or when NYU and UCSD datasets were analyzed separately.
There was a weak trend for greater septal volume with epilepsy duration in patients with ETE (r = 0.38, p = 0.095), and this trend became stronger when TLVV was included as an additional covariate (r = 0.45, p = 0.056).
DISCUSSION
Results demonstrate that patients with TLE have larger septal nuclei than controls. Septal enlargement in TLE is limited to patients without MTS; septal volume in patients with TLE with MTS does not differ from that in controls. The specificity of our results—that septal nuclei are enlarged only in TLE and not in extratemporal epilepsy—is in accord with the known anatomic specificity of septal nuclei connections with hippocampi.1,2
Our results contrast with multiple prior MRI studies (none of which assessed septal nuclei) showing atrophy of hippocampi and interconnected limbic structures in TLE both with and without MTS, considered to reflect neuronal loss in the seizure focus and regions of propagated seizure activity.10,11 Hippocampal seizures propagate to septal nuclei,4,16 so how can we explain our finding of septal enlargement rather than atrophy in patients with TLE without MTS?
A potential explanation comes from animal studies demonstrating that septal cholinergic neurons are resistant to seizure-induced neuronal loss8,17 and may actually enlarge in TLE via a mechanism dependent upon nerve growth factor (NGF) released from hippocampus and transported to septal nuclei retrogradely along the fornix.18,19 NGF is an essential growth factor for septal cholinergic neurons, and its production is highly upregulated by hippocampal seizures.20 While neuronal cell body enlargement does not correspond directly to regional enlargement detectable by MRI, we believe this unusual propensity of septal cholinergic neurons to survive and enlarge in an animal model of TLE provides a relevant model for understanding our MRI finding of septal enlargement in human TLE.
This translational framework also offers an explanation for septal enlargement limited to patients with TLE without MTS: severe hippocampal cell loss might preclude production of sufficient NGF in response to seizures to cause septal enlargement. In support of this idea, septal atrophy occurs following hippocampal excitotoxic ablation21 or septo-hippocampal disconnection.19
In the context of these prior animal studies, as well as studies of human tissue obtained at temporal lobectomy indicating increased septal cholinergic innervation of hippocampus in TLE,22–24 we suggest our finding of septal enlargement in human TLE without MTS represents MRI-detectable evidence of neuroplasticity/augmentation of the septal-hippocampal system in TLE.
Understanding the mechanism, consequences, and cellular basis of septal enlargement in human TLE without MTS will require additional studies. Histologic examination of septal tissue obtained at autopsy or noninvasive imaging tests such as neuroreceptor PET using cholinergic and other ligands can clarify the cellular basis of MRI-detected septal enlargement. Longitudinal studies will be important to ascertain whether septal enlargement is cause, consequence, or both of TLE. In this respect, our results showing no effect of epilepsy duration on septal volume in patients with TLE suggests that this process may occur early in TLE, and our finding of a trend toward larger septal nuclei with longer duration of extratemporal epilepsy could perhaps indicate progression of ETE to involve the hippocampus and the septo-hippocampal system over time.25
Given the role of septo-hippocampal projection neurons in dampening hippocampal hyperexcitability and stopping seizures,4–8 a better understanding of septal nuclei in TLE could open up new antiepileptic therapeutic avenues such septal stimulation. Septal stimulation can abort seizures in animals,6 and stimulation of septal outflow tract in humans is safe, and may improve memory.26
Methodologic issues
The main findings of our study are based on a combined NYU-UCSD dataset. While combining data across different MRI scanners can be problematic, it has become increasingly common in the era of large, multisite neuroimaging studies,27 and is generally considered appropriate so long as both patients and controls are scanned at each site, and site is included as a covariate in analysis,28 as we have done. In addition, we analyzed each dataset separately, and results were concordant.
Ventricle volume as confound
Septal nuclei location in between and adjacent to lateral ventricles could distort their measurement, due to ventricular enlargement or edge effects.29 By explicitly measuring lateral ventricle volume, we were able to control for this effect. It will be important for future studies, especially those focused on disorders associated with marked ventricular enlargement such as dementia, to also measure and account for ventricle volume when assessing septal volume.
We have demonstrated that septal nuclei are enlarged in patients with TLE without MTS. This initial MRI finding in humans can be understood with reference to animal and human studies demonstrating preservation or expansion of the septal-hippocampal cholinergic systems in TLE. Further investigation of this understudied antiepileptic neural circuit could be relevant to development of new therapeutic interventions for refractory TLE.
ACKNOWLEDGMENT
The authors thank Helen Scharfman, PhD, for comments and discussion.
GLOSSARY
- ETE
extratemporal epilepsy
- FCD
focal cortical dysplasia
- FOV
field of view
- icEEG
intracranial EEG
- MNI
Montreal Neurological Institute
- MTS
mesial temporal sclerosis
- NGF
nerve growth factor
- NYU
New York University
- TE
echo time
- TI
inversion time
- TICV
total intracranial volume
- TLE
temporal lobe epilepsy
- TLE-NL
patients with temporal lobe epilepsy without mesial temporal sclerosis
- TLVV
total lateral ventricle volume
- TR
repetition time
- UCSD
University of California, San Diego
AUTHOR CONTRIBUTIONS
T. Butler: conception and design of study, data analysis, data interpretation, drafted manuscript, revised manuscript. L. Zaborszky: conception and design of study, data analysis, data interpretation, revised manuscript. X. Wang: data analysis including critical methodologic development/implementation, revised manuscript. C.R. McDonald: data acquisition, regulatory approval at her site, data analysis, revised manuscript. K. Blackmon: data acquisition, data interpretation, revised manuscript. B.T. Quinn: study concept or design, analysis or interpretation of data, statistical analysis. J. DuBois: data acquisition, data analysis, revised manuscript. C. Carlson: data acquisition, data analysis, revised manuscript. W.B. Barr: study supervision/mentorship, data interpretation, revised manuscript. J. French: study supervision/mentorship, data interpretation, revised manuscript. R. Kuzniecky: study supervision/mentorship, data interpretation, revised manuscript. E. Halgren: study supervision/mentorship, obtained study funding, data interpretation, revised manuscript. O. Devinsky: study supervision/mentorship, data interpretation, revised manuscript. T. Thesen: conception and design of study, study supervision, obtained regulatory approval and funding, data acquisition, data analysis, data interpretation, revised manuscript.
STUDY FUNDING
Supported by FACES (Finding a Cure for Epilepsy and Seizures) and NIH grants NS057579 (T.B.), NS065838 (C.R.M.), NS056091 (C.R.M.), NS18741 (E.H.), NS44623 (E.H.), and NS023945 (L.Z.).
DISCLOSURE
T. Butler: NIH and FACES funding. L. Zaborszky: NIH funding. X. Wang reports no disclosures. C.R. McDonald: NIH funding. K. Blackmon, B.T. Quinn, and J. DuBois report no disclosures. C. Carlson: Epilepsy Foundation funding. W. B. Barr is a paid consultant to The Brainscope Company. J. French serves as the president of The Epilepsy Study Consortium, a nonprofit organization dedicated to improving the lives of epilepsy patients. Dr. Jacqueline French consults for a large number of pharmaceutical companies, in which all money is paid to The Epilepsy Study Consortium and not to Dr. French personally. The money towards her salary is for work performed by Dr. French on behalf of The Epilepsy Study Consortium. Within the past year, The Epilepsy Study Consortium received payments from the 21 companies listed below. All payments are reported annually and reviewed by NYU's Conflict of Interest Committee: Cyberonics; Cypress Bioscience, Inc.; Eisai Medical Research; Entra Pharmaceuticals; GlaxoSmithKline; Icagen, Inc.; Intranasal/Ikano; Johnson & Johnson; Marinus; Neurotherapeutics; NeuroVista Corporation; Ono Pharma USA, Inc.; Ovation/Lundbeck; Pfizer; Sepracor; SK Life Science; Supernus Pharmaceuticals; Taro; UCB Inc/Schwarz Pharma; Upshire Smith; Valeant. R. Kuzniecky: NIH. E. Halgren: NIH; equity interest in CorTechs Labs, Inc. O. Devinsky reports no disclosures relevant to the manuscript. T. Thesen: NIH, FACES funding. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 1983;10:1185–1201 [DOI] [PubMed] [Google Scholar]
- 2.Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci 1990;13:163–169 [DOI] [PubMed] [Google Scholar]
- 3.Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 1993;364:723–725 [DOI] [PubMed] [Google Scholar]
- 4.Kitchigina VF, Butuzova MV. Theta activity of septal neurons during different epileptic phases: the same frequency but different significance? Exp Neurol 2009;216:449–458 [DOI] [PubMed] [Google Scholar]
- 5.Ferencz I, Kokaia M, Elmer E, Keep M, Kokaia Z, Lindvall O. Suppression of kindling epileptogenesis in rats by intrahippocampal cholinergic grafts. Eur J Neurosci 1998;10:213–220 [DOI] [PubMed] [Google Scholar]
- 6.Miller JW, Turner GM, Gray BC. Anticonvulsant effects of the experimental induction of hippocampal theta activity. Epilepsy Res 1994;18:195–204 [DOI] [PubMed] [Google Scholar]
- 7.Garrido Sanabria ER, Castaneda MT, Banuelos C, Perez-Cordova MG, Hernandez S, Colom LV. Septal GABAergic neurons are selectively vulnerable to pilocarpine-induced status epilepticus and chronic spontaneous seizures. Neuroscience 2006;142:871–883 [DOI] [PubMed] [Google Scholar]
- 8.Colom LV, Garcia-Hernandez A, Castaneda MT, Perez-Cordova MG, Garrido-Sanabria ER. Septo-hippocampal networks in chronically epileptic rats: potential antiepileptic effects of theta rhythm generation. J Neurophysiol 2006;95:3645–3653 [DOI] [PubMed] [Google Scholar]
- 9.Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 2008;42:1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzniecky R, Bilir E, Gilliam F, Faught E, Martin R, Hugg J. Quantitative MRI in temporal lobe epilepsy. Neurology 1999;53:496. [DOI] [PubMed] [Google Scholar]
- 11.Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology 2009;72:1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuzniecky RI. Multimodality MRI in mesial temporal sclerosis: relative sensitivity and specificity. Neurology 1997;49:774–778 [DOI] [PubMed] [Google Scholar]
- 13.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113 [DOI] [PubMed] [Google Scholar]
- 14.Butler T, Blackmon K, Zaborszky L, et al. Volume of the human septal forebrain region is a predictor of source memory accuracy. J Int Neuropsychol Soc 2012;18:157–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekkelund S, Pierree-Jerome C, Mellgren S. Quantitative cerebral MRI in epileptic patients. Acta Neurol Scand 1996;94:378–382 [DOI] [PubMed] [Google Scholar]
- 16.Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci 2009;29:13006–13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correia L, Amado D, Cavalheiro EA, Bentivoglio M. Persistence and atrophy of septal/diagonal band neurons expressing the p75 neurotrophin receptor in pilocarpine-induced chronic epilepsy in the rat. Brain Res 1998;809:288–293 [DOI] [PubMed] [Google Scholar]
- 18.Holtzman D, Lowenstein D. Selective inhibition of axon outgrowth by antibodies to NGF in a model of temporal lobe epilepsy. J Neurosci 1995;15:7062–7070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci 1986;6:2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gall C, Isackson P. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science 1989;245:758–761 [DOI] [PubMed] [Google Scholar]
- 21.Sofroniew MV, Cooper JD, Svendsen CN, et al. Atrophy but not death of adult septal cholinergic neurons after ablation of target capacity to produce mRNAs for NGF, BDNF, and NT3. J Neurosci 1993;13:5263–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kish SJ, Olivier A, Dubeau F, Robitaille Y, Sherwin AL. Increased activity of choline acetyltransferase and acetylcholinesterase in actively epileptic human cerebral cortex. Epilepsy Res 1988;2:227–231 [DOI] [PubMed] [Google Scholar]
- 23.Pennell PB, Burdette DE, Ross DA, et al. Muscarinic receptor loss and preservation of presynaptic cholinergic terminals in hippocampal sclerosis. Epilepsia 1999;40:38–46 [DOI] [PubMed] [Google Scholar]
- 24.Green RC, Blume HW, Kupferschmid SB, Mesulam MM. Alterations of hippocampal acetylcholinesterase in human temporal lobe epilepsy. Ann Neurol 1989;26:347–351 [DOI] [PubMed] [Google Scholar]
- 25.Surges R, Schulze-Bonhage A, Altenmuller D-M. Hippocampal involvement in secondarily generalised seizures of extrahippocampal origin. J Neurol Neurosurg Psychiatry 2008;79:924–929 [DOI] [PubMed] [Google Scholar]
- 26.Laxton AW, Tang-Wai DF, McAndrews MP, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol 2010;68:521–534 [DOI] [PubMed] [Google Scholar]
- 27.Van Horn JD, Toga AW. Multi-site neuroimaging trials. Curr Opin Neurol 2009;22:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. Neuroimage 2008;42:611–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grothe M, Zaborszky L, Atienza M, et al. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer's disease. Cereb Cortex 2010;20:1685–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]


