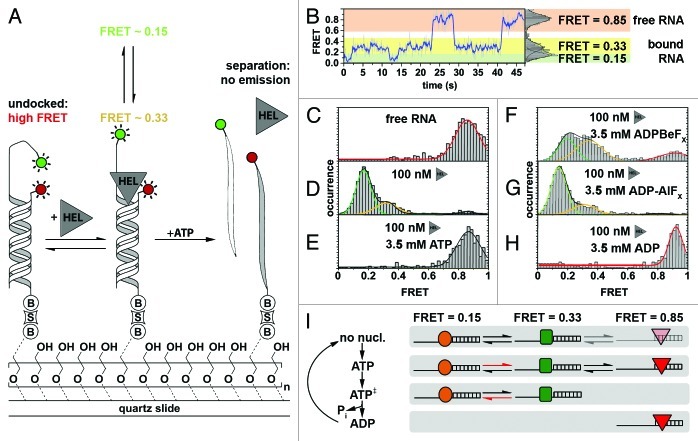
Figure 4. Characterizing NPH-II-catalyzed RNA duplex unwinding. (A) Experimental design and results. A fluorophore-labeled 19-bp RNA duplex (Cy3, green circle; Cy5, red circle) with a 24-nt 3′ extension was immobilized on the PEG-passivated quartz slide via a biotin-streptavidin linkage (“B,” “S”).161 The initial high FRET value of 0.85 is diminished in response to NPH-II binding (“HEL”) and fluctuates between two discrete low FRET values, indicating an increased inter-dye distance. Addition of ATP triggers duplex unwinding ultimately leading to complete loss of emission upon strand separation. (B) Representative smFRET trajectory (1 nM NPH-II, no ATP) showing transition between the helicase-unbound state (FRET 0.85, highlighted in orange), and the helicase-bound states (FRET 0.15 and 0.33, highlighted in green and yellow, respectively). (C–H) Averaged FRET histograms, each built from over 100 individual FRET time traces. Imaging conditions: (C) only RNA, (D) RNA and 100 nM NPH-II, (E) 100 nM NPH-II, 3.5 mM ATP, (F) 100 nM NPH-II, 3.5 mM 'ADP-BeFx (a ground-state analog), (G) 100 nM NPH-II, 3.5 mM ADP-AlFx (a transition state analog), (H) 100 nM NPH-II, 3.5 mM ADP. (I) Basic model for unwinding initiation by NPH-II relying on altered substrate affinities along the ATP hydrolysis cycle. Without nucleotide, NPH-II binds both ssRNA and dsRNA and the NPH-II-ssRNA complex readily alternates between two conformations. ATP binding impedes dissociation from ssRNA and changes the kinetics of bound-state transitions. In the ATP transition state, NPH-II no longer binds to dsRNA and interconversion kinetics change again. The helicase associates with dsRNA upon ATP hydrolysis and phosphate dissociation. Different shapes mark the different conformational states if NPH-II traversed during unwinding initiation. Figure adapted from reference 121.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.