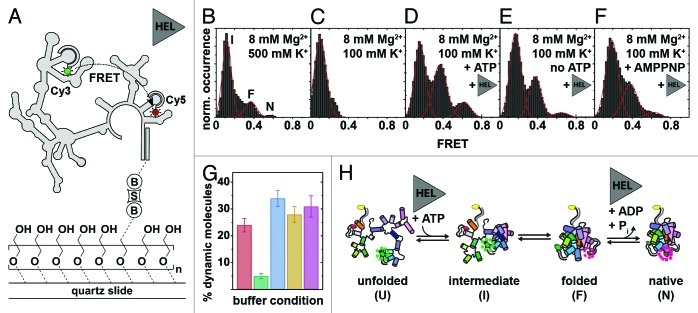
Figure 5. Mss116-mediated group II intron folding using smFRET.124,162 (A) Experimental design. The fluorophore-labeled D135 ribozyme (Cy3, green cycle; Cy5, red cycle) is immobilized on a PEG-coated quartz slide via a biotin-streptavidin linkage (“B,” “S”). Structural interconversion under different folding conditions is monitored by following FRET efficiency over time. (B–F) Averaged FRET histograms, each built from over 100 single-molecule time traces. Imaging conditions: (B) 8 mM Mg2+, 500 mM K+. Three FRET distributions are observed, termed “intermediate” (“I”), “folded” (“F”) and “native” (“N”) based on earlier results.162,163 (C)Eight mM Mg2+, 100 mM K+. Only the “I” FRET state is observed at near-physiological conditions. (D) Eight mM Mg2+, 100 mM K+, 25 nM Mss116, 1 mM ATP. Addition of Mss116 and ATP shifts the distribution of FRET states toward the folded intermediate and the native state. (E and F) Eight mM Mg2+, 100 mM K+, 25 nM Mss116 (and 1 mM AMPPNP). Effect of ATP hydrolysis on D135 folding. Prevalence of the native state is lowered in the absence of ATP (E) and in the presence of non-hydrolyzable AMPPNP (F). (G) Percentage of dynamic molecules at different imaging conditions. Red, 500 mM K+, 8 mM Mg2+; green, 8 mM Mg2+, 100 mM K+; blue, 8 mM Mg2+, 100 mM K+, 25 nM Mss116, 1 mM ATP; yellow, 8 mM Mg2+, 100 mM K+, 25 nM Mss116; purple, 8 mM Mg2+, 100 mM K+, 25 nM Mss116, 1 mM AMPPNP. (H) Proposed model of Mss116-mediated group II intron folding. D135 interconverts between four conformations referred to as “unfolded” (“U”), “intermediate” (“I”), “folded” (“F”) and “native” (“N”). Mss116 promotes the transition from U to I, even in the absence of ATP. It further catalyzes ATP-dependent conversion from F to N. Figure modified from reference 124.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.