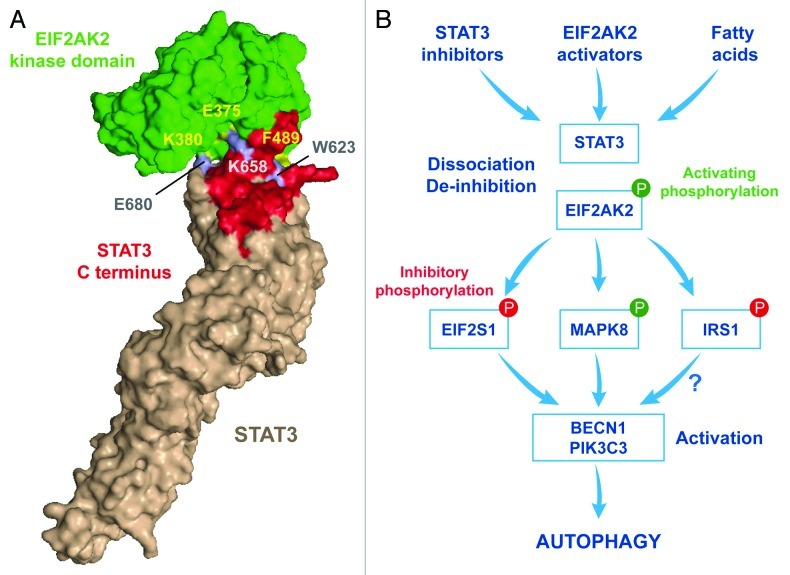
Figure 1. Regulation of fatty acid-induced autophagy by STAT3 and EIF2AK2. (A) In normal conditions, when autophagy is inhibited, cytoplasmic STAT3 and EIF2AK2, engage in a direct inhibitory interaction that appears to involve the following residues: W623, K658 and E680 in STAT3 and E375, K380 and F489 in EIF2AK2. (B) Upon treatment with STAT3 inhibitors, EIF2AK2 activators or fatty acids, the STAT3-EIF2AK2 complex dissociates and EIF2AK2 becomes available to phosphorylate EIF2S1, hence inhibiting translation. EIF2AK2 is also required for the activating phosphorylation of MAPK8, the inhibitory phosphorylation of IRS1, the phosphatidylinositol-3-kinase activity of the BECN1-PIK3C3 complex, and the induction of macroautophagy.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.