Abstract
Background:
Epidemiologic and physiologic studies suggest an association between gastroesophageal reflux disease (GERD) and chronic cough. However, the benefit of antireflux therapy for chronic cough remains unclear, with most relevant trials reporting negative findings. This systematic review aimed to reevaluate the response of chronic cough to antireflux therapy in trials that allowed us to distinguish patients with or without objective evidence of GERD.
Methods:
PubMed and Embase systematic searches identified clinical trials reporting cough response to antireflux therapy. Datasets were derived from trials that used pH-metry to characterize patients with chronic cough.
Results:
Nine randomized controlled trials of varied design that treated patients with acid suppression were identified (eight used proton pump inhibitors [PPIs], one used ranitidine). Datasets from two crossover studies showed that PPIs significantly improved cough relative to placebo, albeit only in the arm receiving placebo first. Therapeutic gain in seven datasets was greater in patients with pathologic esophageal acid exposure (range, 12.5%-35.8%) than in those without (range, 0.0%-8.6%), with no overlap between groups.
Conclusions:
A therapeutic benefit for acid-suppressive therapy in patients with chronic cough cannot be dismissed. However, evidence suggests that rigorous patient selection is necessary to identify patient populations likely to be responsive, using physiologically timed cough events during reflux testing, minimal patient exclusion because of presumptive alternative diagnoses, and appropriate power to detect a modest therapeutic gain. Only then can we hope to resolve this vexing clinical management problem.
Chronic cough, defined as cough that persists for > 8 weeks, affects 11% to 20% of the adult population1 and significantly impairs health-related quality of life,2 leading to substantial socioeconomic burden. Epidemiologic studies suggest an association between gastroesophageal reflux and chronic cough,3 and this relationship is supported by convincing physiologic data. First, in patients with chronic cough, acid infusion into the distal esophagus increases the frequency of coughing4 and cough reflex sensitivity.5 Second, approximately one-half of unselected patients with chronic cough show a positive symptom association between cough and reflux during reflux monitoring.6 However, unlike heartburn, which is usually caused by acid reflux,7 chronic cough has a diverse range of potential causes. Estimates of the proportion of patients with chronic cough in whom reflux is the underlying cause vary greatly among specialists, ranging from 0% to 41%.8 Given the implicit variation in approaches used to identify patients with reflux-related cough, it is perhaps not surprising that a Cochrane review found insufficient evidence to conclude that proton pump inhibitor (PPI) treatment is beneficial in treating nonspecific chronic cough.9
The relationship between gastroesophageal reflux and reflux symptoms is complex in general, but it is particularly complex in the case of chronic cough, in which other disease processes, issues of cause and effect, and hypersensitivity all come into play. Hence, a more thoughtful exploration of the literature may be required to elucidate any treatment benefit for acid-suppressive therapy in this patient group and/or to identify factors that may have prevented studies from detecting benefit with acid-suppressive treatments. For example, with another potential gastroesophageal reflux disease (GERD) syndrome, unexplained chest pain, a recent analysis showed that PPI therapy was effective in patients with objective evidence of GERD (pathologic esophageal acid exposure and/or reflux esophagitis) but not in those without.10 To our knowledge, the impact of this and other variations in study design on therapeutic outcomes for acid-suppressive therapy in patients with chronic cough has not been explored. Thus, the aim of this systematic review was to evaluate the response of chronic cough to acid-suppressive therapy in relation to variations in study design, with a particular focus on distinguishing between studies that included patients with and without objective measures of GERD.
Materials and Methods
Systematic Searches
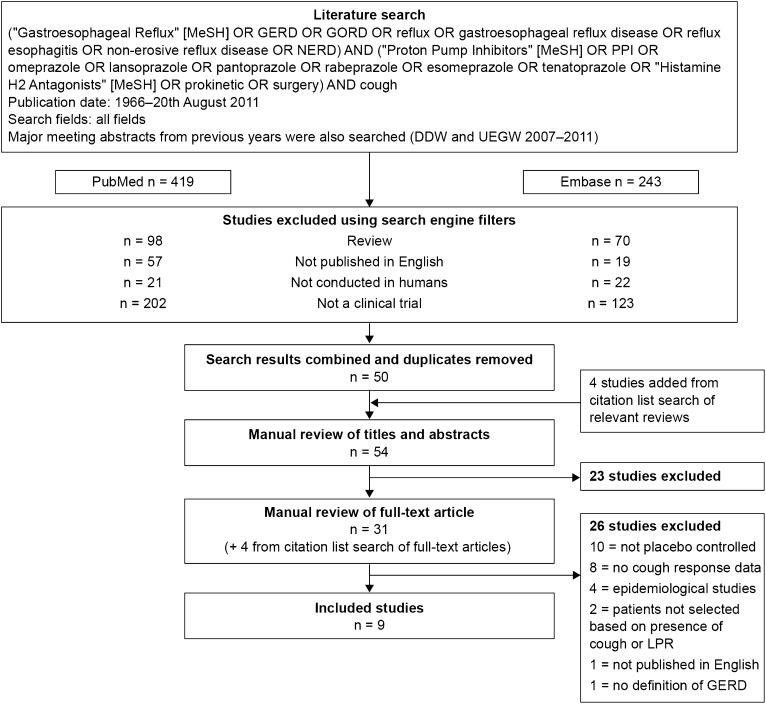
A systematic search of PubMed and Embase (for all years until August 20, 2011) was conducted (Fig 1) as well as a search of recent review articles and abstracts from recent congresses (Digestive Diseases Week, 2008-2011; United European Gastroenterology Week, 2008-2011). Included studies were placebo-controlled clinical trials reporting data on the impact of antireflux therapy on cough in patients selected based on the presence of chronic cough or laryngopharyngeal reflux (LPR), of which cough was a component symptom, and diagnosed with GERD or LPR by objective measures and/or reflux symptoms.
Figure 1.
Summary of the systematic search strategy and study selection process. DDW = Digestive Diseases Week; GERD = gastroesophageal reflux disease; GORD = gastrooesophageal reflux disease; LPR = laryngopharyngeal reflux; NERD = non-erosive reflux disease; PPI = proton pump inhibitor; UEGW = United European Gastroenterology Week.
Reviews, studies not conducted in adult humans, and studies not published in English were excluded using search engine filters. Studies were also excluded if they did not specify the type of acid-suppressive therapy used or if they used a crossover study design without presenting data separately for the first period. The latter exclusion criterion was based on likely period effects for cough (ie, that it tends to improve with time) and the bias associated with carryover effects when an adequate washout period is not used.1 The remaining studies were screened based on titles and abstracts and on the full article when the relevancy of the study was not clear from the abstract.
Analysis of Therapeutic Gain
Where possible, the therapeutic gain associated with acid-suppressive treatment of chronic cough was calculated. This approach was used in recent systematic reviews to compare the therapeutic response of heartburn, regurgitation,11 and unexplained chest pain10 to PPIs across different studies. Therapeutic gain was calculated by subtracting the percentage change from baseline in cough (symptom score or proportion of responders) in the placebo group from that in the treatment group. Second-arm data from crossover studies were not included in the analysis, as discussed previously. Attempts were made to contact study authors for additional information needed for full analysis of their data.12‐16
Results
Systematic Searches and Study Selection
The systematic searches are summarized in Figure 1. Seven studies were excluded for the following reasons: Four were epidemiologic studies,17‐20 one was not published in English,21 and two did not select patients based on the presence of chronic cough or LPR reflux.12,22 A citation list search of the remaining 24 studies identified four additional studies for potential inclusion (28 in total). A placebo control group was not included in 10 of these, and they were excluded.23‐32 Another study was excluded because it did not specify the definition of GERD used.33
Study Characteristics
Characteristics of the nine included studies are summarized in Table 1.13‐16,34‐38 All assessed pharmacologic interventions for cough (no placebo-controlled studies assessing surgery were found); eight assessed PPIs (once daily or bid for 8-16 weeks)13‐16,34‐37; and one assessed the histamine type 2-receptor antagonist ranitidine, 150 mg daily for 8 weeks.38 No unpublished data were used in this review because no additional information that aided the analysis was obtained from authors.
Table 1.
—Description of Included Studies
| Study/Year | Study Inclusion: Chronic Cough and… | Study Exclusion | Treatment Arms | Method of Cough Assessment |
| Ing et al38/1992 | Abnormal pH-metry | Not specified | Ranitidine 150 mg bid (n = 11) Placebo (n = 13) | Diaries: mean change in cough score |
| Havas et al15/1999 | (a) LPR with abnormal pH-metry; (b) LPR with normal pH-metry | Chronic airflow limitation, severe reflux esophagitis | (a) Lansoprazole 30 mg bid (n = 5); placebo (n = 3); (b) Lansoprazole 30 mg bid (n = 3); placebo (n = 4) | Mean change in cough score (frequency × severity) |
| Kiljander et al34/2000 | Abnormal pH-metry | Postnasal drip, asthma, abnormal chest radiograph, smokers | Omeprazole 40 mg (n = 9) Placebo (n = 12) | Mean change in cough score over final 3 wk |
| Noordzij et al35/2001 | LPR with abnormal pH-metry | Infectious laryngitis, laryngeal cancer, allergies | Omeprazole 40 mg bid (n = 15) Placebo (n = 15) | Questionnaire: mean change in cough score (frequency × severity) |
| Ours et al36/1999 | Abnormal pH-metry | Asthma, abnormal chest radiograph, smokers | Omeprazole 40 mg bid (n = 8) Placebo (n = 15) | Diaries: cough score (frequency × severity, day/night) prespecified criteria |
| Shaheen et al37/2011 | (a) Abnormal pH-metry; (b) Normal pH-metry | Postnasal drip, heartburn, abnormal chest radiograph, smokers | (a) Esomeprazole 40 mg bid (n = 10); placebo (n = 7); (b) Esomeprazole 40 mg bid (n = 12); placebo (n = 11) | Fisman cough severity and frequency score; mean change |
| Steward et al14/2004 | LPR symptoms | GI surgery, malignancy | Rabeprazole 20 mg bid (n = 18) Placebo (n = 19) | Mean change in cough score (frequency × severity) |
| Wo et al13/2006 | LPR with abnormal pH-metry | Prior LPR, GERD, or gastric surgery | Pantoprazole 40 mg (n = 20) Placebo (n = 19) | Mean change in cough score |
| Vaezi et al16/2006 | LPR with normal pH-metry | Infectious laryngitis, malignancy, sinusitis | Esomeprazole 40 mg bid (n = 11) Placebo (n = 8) | Diaries: cough severity, prespecified criteria |
GERD = gastroesophageal reflux disease; LPR = laryngopharyngeal reflux.
Methods for assessing cough varied substantially across studies. Five used patient diaries consisting of visual analog scales to assess cough severity and/or frequency (Table 1),13,16,34,36,38 questionnaires were used in two studies (Table 1),14,35 and two studies did not specify the method of data acquisition (Table 1).15,37 Two studies assessed efficacy in terms of the proportion of patients who met prespecified criteria for response (Table 1)16,36; the remainder measured change in mean cough scores relative to baseline for treatment vs placebo. Sample sizes across the studies were 15 to 40, and the sample sizes for placebo and active treatment groups were seven to 19 and eight to 22, respectively.
Reported Analyses for the Response of Chronic Cough to Acid-Suppressive Therapy
Chronic cough was reported to respond significantly to acid-suppressive therapy in two of the nine included trials (Table 1)34,38; both used a crossover design to assess the impact of 8 weeks of acid-suppressive therapy on cough scores in patients with pathologic esophageal acid exposure. In each case, the statistically significant response was only detected for the first period of the crossover study. Another study, which also assessed the impact of 8 weeks of acid-suppressive therapy on cough scores in patients with pathologic esophageal acid exposure but was not a crossover design, reported a trend toward improved cough scores (Table 1).35 Of the six studies that did not report significantly improved cough scores, five used a treatment period of ≥ 12 weeks (Table 1),13,15,16,36,37 three included patients who did not have pathologic esophageal acid exposure (Table 1),15,16,37 and two excluded patients with heartburn (Table 1).16,37
Therapeutic Response of Chronic Cough to Acid-Suppressive Therapy
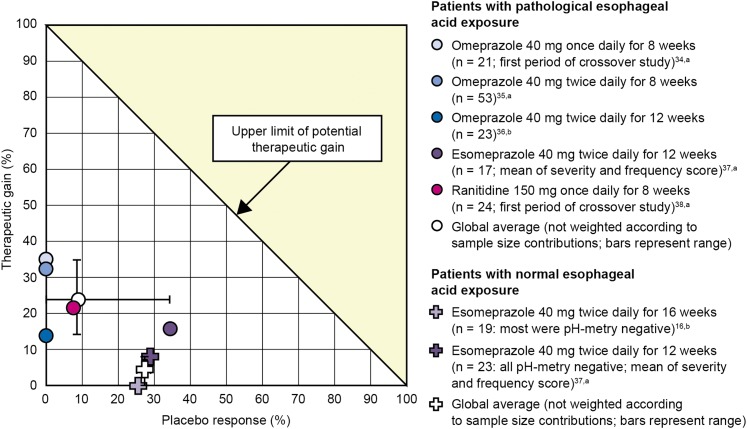
Sufficient data for calculating the therapeutic gain of acid-suppressive therapy were available in six studies (Table 1).16,34‐38 Shaheen et al37 (Table 1) reported data separately for patients with and without pathologic esophageal acid exposure and, thus, contributed two datasets, resulting in a total of seven datasets for further analysis. One dataset showed no therapeutic gain (Table 1).16 Therapeutic gains across the six remaining datasets were 8.6% to 35.8% (nonweighted mean, 21.5%).
Patients With Pathologic Esophageal Acid Exposure:
The five datasets with the greatest therapeutic gain were those that included only patients with pathologic esophageal acid exposure. The range was 12.5% to 35.8% (nonweighted mean, 24.1%) (Fig 2). The three with the greatest therapeutic gain in this group all assessed treatment efficacy in terms of the change in cough score from baseline after 8 weeks of therapy. The lowest therapeutic gain was observed for ranitidine.38 The two datasets with the lowest therapeutic gain both used a treatment period of 12 weeks. In addition, one assessed efficacy in terms of the proportion of patients meeting prespecified response criteria (Table 1),36 and one excluded patients with heartburn (Table 1).37
Figure 2.
Calculated therapeutic gain for datasets derived from patients with pathologic esophageal acid exposure and populations including patients with normal esophageal acid exposure. aPercentage change in symptom score; bPercentage change in proportion of responders.
Patients Without Pathologic Esophageal Acid Exposure:
The two lowest therapeutic gains were 0.0% and 8.6% and were observed in the only two datasets (Table 1)16,37 that selected patients with normal esophageal acid exposure (Fig 2). Both also excluded patients with heartburn. In addition, the dataset exhibiting no therapeutic gain was after 16 weeks of treatment (the longest treatment period across all studies) and was calculated based on the proportion of responders meeting prespecified response criteria.
Placebo Response of Chronic Cough to Therapy
The mean placebo response rate across all seven datasets was 13.8%. The greatest placebo response rate was 33.5% and was from the Shaheen et al37 study that included only patients with pathologic esophageal acid exposure. This was similar to the placebo response rate of 29.5% observed in patients without pathologic esophageal acid exposure from the same study. However, the mean placebo response rate across the five datasets that included only patients with pathologic esophageal acid exposure was less than the mean placebo response rate across the two datasets that included patients with normal esophageal acid exposure (8.4% vs 24.1%) (Fig 2).
Discussion
Reflux-cough syndrome is something of an enigma, in that despite substantial uncontrolled data, well summarized in the American College of Chest Physicians 2006 Practice Guidelines, suggesting efficacy of diet, antacids, histamine-2 receptor antagonists, prokinetics, PPIs, and antireflux surgery in improving or curing reflux cough syndrome,39 controlled trials have consistently failed to demonstrate this.39 Hence, we aimed to systematically review studies assessing the response of chronic cough to acid-suppressive therapy, with a focus on relating study outcomes to differences in study design and whether the patients studied had objective evidence of GERD. Of the nine placebo-controlled, randomized clinical trials identified, only two reported a statistically significant reduction in cough frequency and/or severity after pharmacologic acid-suppressive therapy. However, in six34‐38 of the seven datasets in which therapeutic gain could be calculated, acid-suppressive treatment had a greater effect than placebo. The only dataset demonstrating no therapeutic gain16 was from a study that enrolled patients with normal esophageal pH-metry. When therapeutic gains were classified according to pH-metry characteristics, they were greater in patients with pathologic esophageal acid exposure (range, 12.5%-35.8%)34‐38 than in those without (range, 0.0%-8.6%).16,37
The suggestion that acid suppression may be beneficial in treating chronic cough in patients with GERD begs the question of why so few studies were able to detect a statistically significant effect. Clearly, small sample sizes may be an issue. Indeed, most studies appear to be powered to detect a therapeutic benefit for acid suppression of the magnitude that might be expected for heartburn, regurgitation,11 or unexplained chest pain.10 However, for reasons discussed later, a therapeutic benefit of that magnitude is unlikely for chronic cough, even in patients with pathologic esophageal acid exposure. And, of course, even though our findings hint at a relatively consistent effect for acid-suppressive therapy relative to placebo on cough in patients with pathologic esophageal acid exposure, they should be viewed with caution given the absence of sufficient data for meta-analysis.
If acid-suppressive treatment does have a therapeutic benefit in some patients with chronic cough, it is clear that the effect is substantially less than that observed in patients with GERD for heartburn, regurgitation, or unexplained chest pain,10,11 despite the use of pH-metry to enrich the study population with patients with reflux. The elegant study by Smith et al6 provides insight into why patients with chronic cough are relatively refractory to GERD therapy. That study combined sound recordings with acoustic analysis and pH-impedance recordings to accurately define the temporal relationship between cough and reflux events. Of the unselected patients with chronic cough assessed, 48% had a positive symptom association probability for cough preceded by reflux. Even more importantly, 56% of patients had a positive symptom association probability for reflux preceded by cough, and 32% had a positive symptom association probability in both directions. The authors concluded that in addition to reflux potentially inducing cough, cough could also induce reflux, possibly by increasing transient lower esophageal sphincter relaxations (considered a major mechanism for generating reflux events40,41) via a CNS mechanism. Furthermore, it was shown that many patients had cough associated with both acid and weakly acidic reflux. Finally, building upon earlier experimental observations of increased sensitivity of the cough reflex with esophagitis42 and an acute lowering of the tussigenic threshold to inhaled capsaicin by esophageal acid perfusion in patients with GERD irrespective of whether they had chronic cough,5 Smith and colleagues observed increased sensitivity to a tussigenic challenge in the subset of patients with cough preceded by reflux. Together, these observations strongly implicate increased cough sensitivity as an important operant mechanism in patients with reflux cough.
The implications of the findings of Smith et al6 for the current study are twofold. First, many patients with chronic cough may have pathologic esophageal acid exposure because of acid reflux induced by coughing. Hence, although they have both conditions, the chronic cough may still be caused by other factors and, consequently, be unresponsive to acid suppression. Second, even in patients whose cough is caused by reflux, weakly acidic reflux resulting from acid-suppressive therapy may still be sufficient to perpetuate cough. The apparent success of antireflux surgery in treating patients with chronic cough refractory to PPI therapy, albeit in uncontrolled trials, supports the concept that weakly acidic reflux may be sufficient to perpetuate chronic cough in some patients.39,43 Both of these factors and the observed hypersensitivity of the cough reflex would lead to a diminution of the therapeutic response observed in clinical trials of acid suppression in patients with chronic cough if pathologic esophageal acid exposure was the only coselection criterion. Conversely, the therapeutic response rate for acid-suppressive therapy for patients with chronic cough may be improved by adopting the methodology of Smith et al6 to select only patients with a significant symptom association pattern for cough preceded by acid reflux. Obviously, no such trial has yet been conducted. However, an indication that more stringent patient selection criteria might improve response rates was provided in the observational study by Hersh et al.44 Despite less precise methodology than that used by Smith et al,6 Hersh et al44 reported that patients with a positive symptom association pattern for cough and reflux (60% of the series) had a better response to antireflux therapy compared with patients with pathologic esophageal acid exposure but negative symptom association (44% of the series). Surgery was also included among antireflux therapies in the Hersh et al44 study. Antireflux surgery may be more effective than acid-suppressive therapy in patients with chronic cough, as both acid and weakly acidic reflux should be reduced. Consistent with our findings, a recent retrospective analysis concluded that this was particularly true in patients with concomitant heartburn or > 12% esophageal acid exposure on pH-metry.45 However, among the many studies reporting benefit in reduced chronic cough after antireflux surgery,23,24,27,32 none were placebo controlled, which is essential for a condition that tends to resolve naturally over time.
Apart from verifying objective evidence of reflux disease, our findings suggest other design aspects to consider in future trials. Clearly, a crossover design should be avoided both because of a potential spontaneous recovery and because of carryover effect. With respect to trial duration, 8 weeks seemed optimal, as the best responses were observed in trials of that duration, with longer trials actually exhibiting less treatment effect. The cough outcome measure is also a key aspect of study design. Complete resolution of cough, although desirable, was rarely achieved, and studies stipulating arbitrary, strict cutoff values to define patient response had few, if any, responders.16,36 More reasonable would be to use a validated cough assessment instrument linking the definition of a response to a patient-defined meaningful improvement in quality of life. Finally, the two studies with the lowest therapeutic gain for acid-suppressive therapy were also the only two studies to exclude patients with heartburn.16,37 Including patients with pathologic esophageal acid exposure who do not have heartburn likely selects for patients with low esophageal sensitivity, as suggested in a recent comprehensive review of reflux perception.7 Given that esophageal sensitivity may play a role in reflux-induced cough, these criteria may inadvertently select against likely responders.
In conclusion, a therapeutic benefit for acid-suppressive therapy in patients with chronic cough could not be dismissed. However, evidence reviewed suggests that rigorous patient selection is likely necessary to identify responsive patient populations. Future trials of GERD therapy for chronic cough should include patients irrespective of concomitant conditions such as asthma or post-nasal drip who have a positive reflux-cough association determined by physiologically timed cough events during reflux testing. Clinical efficacy should be assessed after 8 weeks’ treatment and should be based on a clinically meaningful, but not necessarily complete, improvement in symptom score relative to baseline. Finally, studies need to be sufficiently powered to detect a therapeutic gain in the range of 20% to 30%. Only then can we hope to resolve this vexing clinical management problem.
Acknowledgments
Author contributions: Dr Kahrilas is the guarantor of this study.
Dr Kahrilas: contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and independently screening the search results and approving the final draft of the manuscript.
Dr Howden: contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and independently screening the search results and approving the final draft of the manuscript.
Dr Hughes: contributed to conceiving and writing the manuscript, data analysis, and independently screening the search results and approving the final draft of the manuscript.
Dr Molloy-Bland: contributed to conceiving and writing the manuscript, data analysis, and independently screening the search results and approving the final draft of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kahrilas has acted as a consultant for EndoGastric Solutions; Given Imaging Ltd; Ironwood Pharmaceuticals, Inc; and Torax Medical, Inc. Dr Howden has acted as a consultant for Takeda Pharmaceutical Company Limited; XenoPort, Inc; Santarus, Inc; Procter & Gamble; Merck/Schering-Plough Pharmaceuticals; Boehringer Ingelheim GmbH; Novartis Consumer Health, Inc; Novartis Oncology; Otsuka Pharmaceutical Co, Ltd; and KV Pharmaceutical Company and as a speaker for Takeda Pharmaceutical Company Ltd, Novartis AG, GlaxoSmithKline, and Otsuka Pharmaceutical Co, Ltd. Dr Hughes is an employee of Oxford PharmaGenesis Ltd, which received funding from AstraZeneca R&D Mölndal, Sweden. Dr Molloy-Bland is an employee of Oxford PharmaGenesis Ltd, which received funding from AstraZeneca R&D Mölndal, Sweden.
Role of sponsors: AstraZeneca R&D contributed to the development of the initial study concept and had the opportunity to comment on manuscript drafts. The direction of the manuscript and final content was determined by the authors.
Abbreviations
- GERD
gastroesophageal reflux disease
- LPR
laryngopharyngeal reflux
- PPI
proton pump inhibitor
Footnotes
Funding/Support: This study was supported by funding from AstraZeneca R&D Mölndal, Sweden. Dr Kahrilas is supported by Public Health Service [Grant R01 DK56033].
For editorial comment see page 587
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ. 2006;332(7532):11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657-1661 [DOI] [PubMed] [Google Scholar]
- 3.Irwin RS, Richter JE. Gastroesophageal reflux and chronic cough. Am J Gastroenterol. 2000;95(suppl 8):S9-S14 [DOI] [PubMed] [Google Scholar]
- 4.Ing AJ, Ngu MC, Breslin AB. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med. 1994;149(1):160-167 [DOI] [PubMed] [Google Scholar]
- 5.Javorkova N, Varechova S, Pecova R, et al. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterol Motil. 2008;20(2):119-124 [DOI] [PubMed] [Google Scholar]
- 6.Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139(3):754-762 [DOI] [PubMed] [Google Scholar]
- 7.Bredenoord AJ. Mechanisms of reflux perception in gastroesophageal reflux disease: a review. Am J Gastroenterol. 2012;107(1):8-15 [DOI] [PubMed] [Google Scholar]
- 8.Morice AH, Fontana GA, Sovijarvi AR, et al. ERS Task Force The diagnosis and management of chronic cough. Eur Respir J. 2004;24(3):481-492 [DOI] [PubMed] [Google Scholar]
- 9.Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2006; (4):CD004823. [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas P, Hughes N, Howden C. Response of unexplained chest pain to proton pump inhibitor treatment in patients with and without objective evidence of gastro-oesophageal reflux disease. Gut. 2011;60(11):1473-1478 [DOI] [PubMed] [Google Scholar]
- 11.Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106(8):1419-1425 [DOI] [PubMed] [Google Scholar]
- 12.Teichtahl H, Kronborg IJ, Yeomans ND, Robinson P. Adult asthma and gastro-oesophageal reflux: the effects of omeprazole therapy on asthma. Aust N Z J Med. 1996;26(5):671-676 [DOI] [PubMed] [Google Scholar]
- 13.Wo JM, Koopman J, Harrell SP, Parker K, Winstead W, Lentsch E. Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol. 2006;101(9):1972-1978 [DOI] [PubMed] [Google Scholar]
- 14.Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131(4):342-350 [DOI] [PubMed] [Google Scholar]
- 15.Havas T, Huang S, Levy M, et al. Posterior pharyngolaryngitis: double-blind randomised placebo-controlled trial of proton pump inhibitor therapy. Aust J Otolaryngol.. 1999;3(3):243-246 [Google Scholar]
- 16.Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116(2):254-260 [DOI] [PubMed] [Google Scholar]
- 17.Jaspersen D, Kulig M, Labenz J, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther. 2003;17(12):1515-1520 [DOI] [PubMed] [Google Scholar]
- 18.Jaspersen D, Labenz J, Willich SN, et al. Long-term clinical course of extra-oesophageal manifestations in patients with gastro-oesophageal reflux disease. A prospective follow-up analysis based on the ProGERD study. Dig Liver Dis. 2006;38(4):233-238 [DOI] [PubMed] [Google Scholar]
- 19.Jaspersen D, Nocon M, Labenz J, et al. Clinical course of extra-esophageal disorders in gastroesophageal reflux disease during routine care: a 5-year follow-up study [Abstract]. Gastroenterol. 2009;136(5)(suppl 1):A-739 [Google Scholar]
- 20.Yoshida S, Nii M, Date M. Effects of omeprazole on symptoms and quality of life in Japanese patients with reflux esophagitis: final results of OMAREE, a large-scale clinical experience investigation. BMC Gastroenterol. 2011;1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavazzoni FB, de Ataíde AL, Herrero Júnior FH, de Macedo Filho ED. Reflux esophagitis and reflux laryngitis: different stages of the same disease?. Braz J Otorhinolaryngol. 2002;68(1):86-90 [Google Scholar]
- 22.Pawar S, Lim HJ, Gill M, et al. Treatment of postnasal drip with proton pump inhibitors: a prospective, randomized, placebo-controlled study. Am J Rhinol. 2007;21(6):695-701 [DOI] [PubMed] [Google Scholar]
- 23.Allen CJ, Anvari M. Gastro-oesophageal reflux related cough and its response to laparoscopic fundoplication. Thorax. 1998;53(11):963-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen CJ, Anvari M. Preoperative symptom evaluation and esophageal acid infusion predict response to laparoscopic Nissen fundoplication in gastroesophageal reflux patients who present with cough. Surg Endosc. 2002;16(7):1037-1041 [DOI] [PubMed] [Google Scholar]
- 25.Anvari M, Allen C, Goldsmith C. A randomized controlled trial of laparoscopic nissen fundoplication (LNF) versus proton pump inhibitors for treatment of patients with chronic gastro-esophageal reflux disease (GERD) who complained of cough [abstract]Gastroenterol. 2010;138(5)(suppl 1):S-885.
- 26.Baldi F, Cappiello R, Cavoli C, Ghersi S, Torresan F, Roda E. Proton pump inhibitor treatment of patients with gastroesophageal reflux-related chronic cough: a comparison between two different daily doses of lansoprazole. World J Gastroenterol. 2006;12(1):82-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwer R, Kiroff GK. Improvement of respiratory symptoms following laparoscopic Nissen fundoplication. ANZ J Surg. 2003;73(4):189-193 [DOI] [PubMed] [Google Scholar]
- 28.Dore MP, Pedroni A, Pes GM, et al. Effect of antisecretory therapy on atypical symptoms in gastroesophageal reflux disease. Dig Dis Sci. 2007;52(2):463-468 [DOI] [PubMed] [Google Scholar]
- 29.Eubanks TR, Omelanczuk P, Hillel A, Maronian N, Pope CE, Pellegrini CA. Pharyngeal pH measurements in patients with respiratory symptoms before and during proton pump inhibitor therapy. Am J Surg. 2001;181(5):466-470 [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Téllez M, Galera-Ruiz H, Argüelles-Arias F, Carmona I, Muñoz-Borje F, Herrerías JM. Posterior laryngitis: effects of treatment with omeprazole alone. Rev Esp Enferm Dig. 2002;94(3):123-130 [PubMed] [Google Scholar]
- 31.Toros AB, Toros SZ, Ozel L, Ersoz F, Saglam M, Sametoglu F. Comparative outcomes of antireflux treatment for laryngopharyngeal reflux symptoms and upper abdominal symptoms in patients with endoscopic esophagitis. Eur Arch Otorhinolaryngol. 2011;268(5):703-708 [DOI] [PubMed] [Google Scholar]
- 32.Ziora D, Jarosz W, Dzielicki J, et al. Citric acid cough threshold in patients with gastroesophageal reflux disease rises after laparoscopic fundoplication. Chest. 2005;128(4):2458-2464 [DOI] [PubMed] [Google Scholar]
- 33.Kopec SE, Irwin RS, French CL, Wilson MM, Bol S. A double-blind randomized placebo-controlled trial comparing diet and/or cisapride [abstract]. Am J Respir Crit Care Med. 2001;163(suppl 5):A64 [Google Scholar]
- 34.Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Chronic cough and gastro-oesophageal reflux: a double-blind placebo-controlled study with omeprazole. Eur Respir J. 2000;16(4):633-638 [DOI] [PubMed] [Google Scholar]
- 35.Noordzij JP, Khidr A, Evans BA, et al. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope. 2001;111(12):2147-2151 [DOI] [PubMed] [Google Scholar]
- 36.Ours TM, Kavuru MS, Schilz RJ, Richter JE. A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol. 1999;94(11):3131-3138 [DOI] [PubMed] [Google Scholar]
- 37.Shaheen NJ, Crockett SD, Bright SD, et al. Randomised clinical trial: high-dose acid suppression for chronic cough - a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(2):225-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ing AJ, Ngu MC, Breslin ABX. A randomised double blind placebo controlled cross-over study of ranitidine in patients with chronic persistent cough (CPC) associated with gastro-oesophageal reflux (GOR) [abstract]. Am Rev Resp Dis.. 1992;145(4 pt 2):A11 [Google Scholar]
- 39.Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):80S-94S [DOI] [PubMed] [Google Scholar]
- 40.Dent J, Holloway RH, Toouli J, Dodds WJ. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut. 1988;29(8):1020-1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holloway RH, Dent J. Pathophysiology of gastroesophageal reflux. Lower esophageal sphincter dysfunction in gastroesophageal reflux disease. Gastroenterol Clin North Am. 1990;19(3):517-535 [PubMed] [Google Scholar]
- 42.Benini L, Ferrari M, Sembenini C, et al. Cough threshold in reflux oesophagitis: influence of acid and of laryngeal and oesophageal damage. Gut. 2000;46(6):762-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg. 2006;93(12):1483-1487 [DOI] [PubMed] [Google Scholar]
- 44.Hersh MJ, Sayuk GS, Gyawali CP. Long-term therapeutic outcome of patients undergoing ambulatory pH monitoring for chronic unexplained cough. J Clin Gastroenterol. 2010;44(4):254-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francis DO, Goutte M, Slaughter JC, et al. Traditional reflux parameters and not impedance monitoring predict outcome after fundoplication in extraesophageal reflux. Laryngoscope. 2011;121(9):1902-1909 [DOI] [PubMed] [Google Scholar]