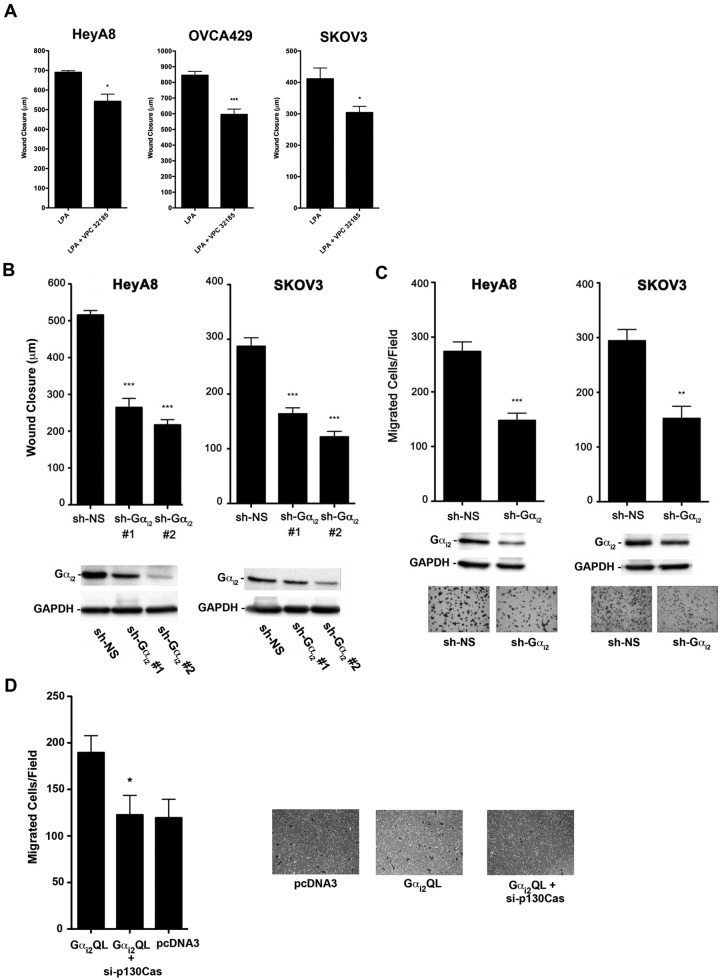
Figure 7.
Inhibition of the LPAR1/3-Gαi2-p130Cas signaling axis attenuates LPA-stimulated migration of ovarian cancer cells. (A) Role of LPAR1/3 in LPA-stimulated cell migration. HeyA8 (5 × 105), SKOV3 (7 × 105), and OVCA429 (7 × 105) cells were plated in 6-well plates and allowed to grow to confluence. The cells were then serum starved overnight and treated with mitomycin C (10 µg/mL) to inhibit proliferation, and then 3 separate wounds were made per well. Pictures were taken at 3 random fields per wound and marked, and the cells were then stimulated with 20 µM of LPA or 20 µM of LPA plus 10 µM VPC32183 for 24 hours. After 24-hour stimulation, pictures were taken of the same location, and cell movement was measured with the CellSens program. *P < 0.05. ***P < 0.001 (n = 3). (B) Silencing Gαi2 attenuates LPA-stimulated migration of ovarian cancer cells. Two different shRNAs that target different regions of Gαi2 or nonsense shRNA were transiently transfected into HeyA8 and SKOV3 (2 × 106) cells. The wound closure assay was carried out at 48 hours following transfection as described in Figure 6A. Silencing of Gαi2 in these cells was verified by immunoblot analysis (lower panel). (C) Silencing Gαi2 attenuates LPA-stimulated invasive migration of ovarian cancer cells. HeyA8 and SKOV3 (2 × 106) cells were transfected with Gαi2-specific shRNA or nonsense shRNA. Silencing of Gαi2 in these cells was verified by immunoblot analysis (lower panel). The transwell assay was carried out as described in Figure 6C. Representative images from each experimental group are shown. (D) Gαi2-stimulated invasive migration is dependent on p130Cas. HeyA8 cells were transiently transfected with Gαi2Q205L, Gαi2Q205L + si-p130Cas, or with vector alone (pcDNA3). The cells were allowed to grow in 60-mm plates for approximately 48 hours. There were 2.5 × 104 cells counted and suspended in 200 mL of serum-free media and subsequently plated on collagen-1–coated transwell inserts in 24-well plates with 500 mL of serum-free media. The cells were incubated at 37°C and allowed to migrate for 20 hours. The transwells were then processed as described in Figure 6C. The experiments were repeated at least 3 times, and the results presented are from a typical experiment. *P < 0.05. **P < 0.01. ***P < 0.001 (n = 3).