Figure 1.
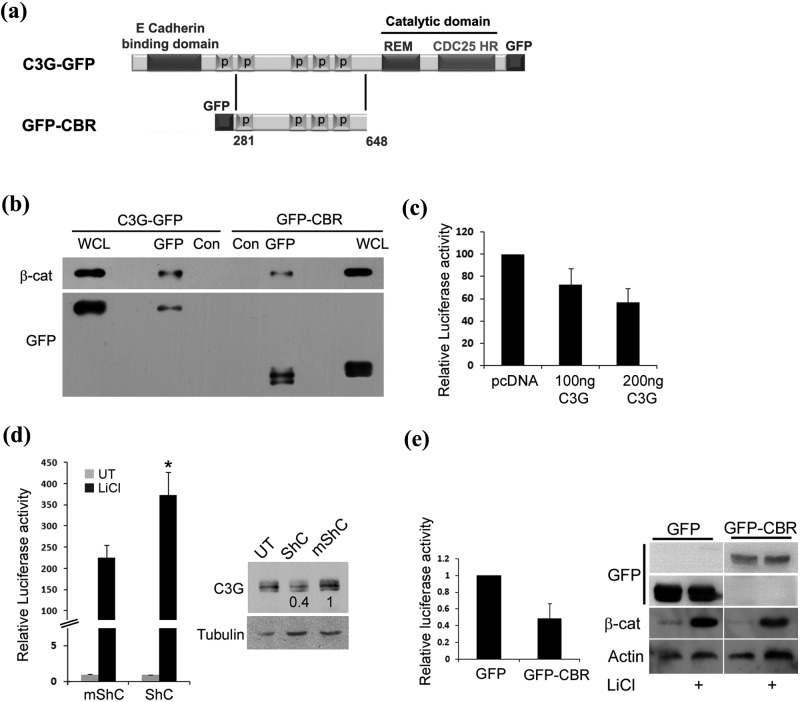
C3G interacts with and downregulates transcription activity of β-catenin/TCF. (a) Schematic shows domains of C3G in C3G-GFP and GFP-CBR constructs. (b) Cellular β-catenin co-precipitates with C3G. Cell lysates of HEK 293T cells transfected with C3G-GFP or GFP-CBR were subjected to immunoprecipitation with either GFP antibody or normal mouse IgG (Con), followed by Western blotting to detect the indicated proteins with the corresponding antibodies. (c) Effect of C3G on transcription activity of β-catenin induced by GSK3β inhibition. Lysates of HEK 293T cells transfected with reporter constructs along with pcDNA or C3G expression vector and treated with LiCl were used for luciferase assays. Relative luciferase activity after normalization with β-Gal is shown as mean ± SD. (d) Knockdown of endogenous C3G levels causes enhanced β-catenin dependent promoter activation. HEK 293T cells transfected with reporter constructs along with shRNA construct targeting C3G (ShC) or mutant shRNA (mShC) were either left untreated or treated with LiCl. Cell lysates, made after 48 hours of expression, were used for luciferase assays and Western blotting to detect C3G (right panel). Tubulin was used as a loading control. Relative luciferase activity after normalization with β-Gal is shown as mean ± SD in the bar diagram.*P < 0.01. (e) Crk binding region (CBR) of C3G is sufficient for repressing TCF activity. β-catenin dependent luciferase assay in HEK 293T cells expressing GFP or GFP-CBR and treated with LiCl. Western blot was carried out using the lysates to confirm an increase in β-catenin levels upon LiCl treatment. Expression of GFP and GFP-CBR was detected by probing the corresponding regions of the blot with GFP antibody.