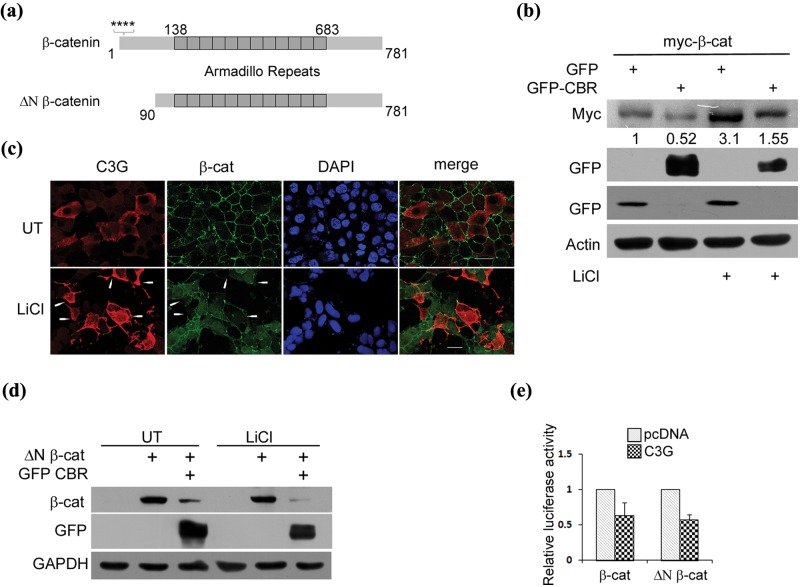
Figure 3.
C3G represses β-catenin independent of GSK3β. (a) A schematic showing full-length β-catenin and constitutively active ΔN β-catenin. Stars indicate serine residues phosphorylated by CK1 and GSK3β. (b) CBR represses exogenously expressed β-catenin protein under conditions of GSK3β inhibition. Lysates of HEK 293T cells expressing myc tagged full-length β-catenin along with GFP or GFP-CBR and treated with LiCl were subject to Western blotting to detect the indicated proteins with antibodies. (c) C3G overexpressing cells showed reduced staining for cellular β-catenin induced by LiCl treatment. HEK 293T cells transfected with pcDNA C3G were treated with 50 mM LiCl after 6 hours of expression and fixed after 30 hours of expression followed by indirect immunofluorescence to detect C3G and β-catenin expression. Arrows indicate some C3G expressing cells. The C3G antibody used does not detect endogenous C3G under the conditions of our experiment. Bar, 20 µm. (d) CBR domain of C3G represses β-catenin protein lacking its N-terminal regulatory domain. ΔN β-catenin protein levels were examined in HEK 293T cells co-expressing pcDNA or GFP-CBR in the presence or absence of LiCl. (e) C3G inhibits activity of ΔN β-catenin. Lysates of HEK 293T cells transfected with reporter constructs and 100 ng of pcDNA or C3G along with 100 ng of β-catenin or ΔN β-catenin expression vectors were used for luciferase assays. Relative luciferase counts after normalization with β-Gal are shown as mean ± SD in the bar diagram.