Figure 3.
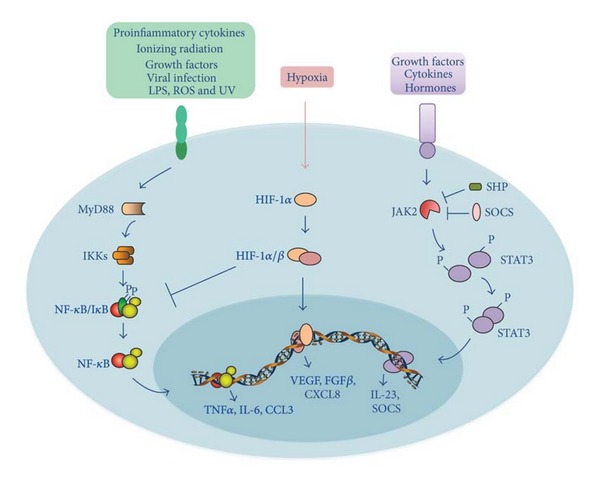
Schematic representation of NF-κB, HIF-1, and STAT-3 signaling pathways linking inflammation and liver cancer. Proinflammatory signals bind to their corresponding receptors, leading to the recruitment of receptor-associated proteins, such as MyD-88. In turn, these associated proteins trigger a phosphorylation cascade that leads to activation of the IKK complex, which is responsible for the phosphorylation of the IκB protein. Phosphorylated IκB is degraded by proteasome, thereby allowing free NF-κB dimers to translocate to the nucleus and transactivate target genes, such as TNFα, IL-6, and CCL3. HIF-1α is the central regulator of the hypoxic response. HIF-1α is activated by hypoxia and its activity progressively increases with a decrease in O2 gradient. Heterodimerization of HIF-1α with HIF-1β allows for DNA binding to hypoxia response elements (HRE) and transactivation of its target genes, such as VEGF, FGFβ, and CXCL8. Cytokine, hormone and growth factor stimulation activates JAK2, which in turn phosphorylates STAT3, allowing its dimerization, nuclear translocation, and transactivation of target genes, such as IL-23. STAT-3 signaling is turned off by protein inhibitors, such as SHP and SOCS, which are induced by STAT-3 in a negative feedback loop. Refer to the text for abbreviations.
