Figure 4. Representative conformations of Bcl-XL .
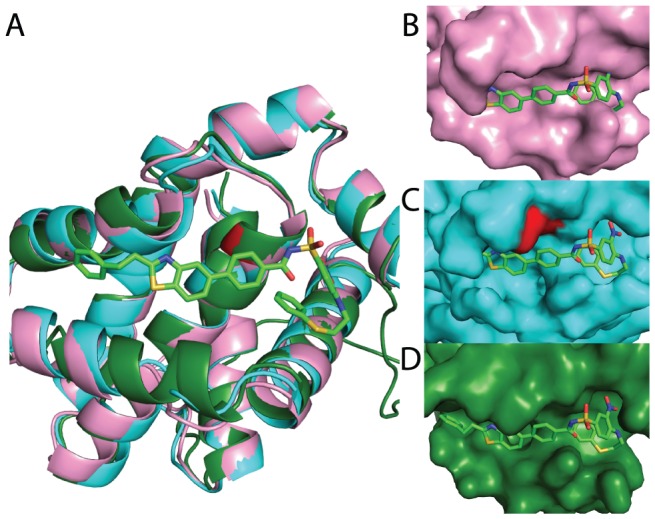
. An unbound crystal structure (pink), an inhibitor-bound crystal structure (green, with inhibitor shown in sticks), and a low-energy conformation generated from the unbound crystal structure using the biasing potential (cyan, with target residue in red) are shown. (A) The overall protein architecture is preserved amongst all three; movement of the helix in the foreground upon binding is not recapitulated in the pocket-opened conformation. (B–D) The pocket revealed in this low-energy conformation nonetheless strongly resembles the surface pocket in the bound crystal structure, and even bears shape-complementarity to the inhibitor. The identity of the inhibitor was not used in generating this conformation, but was added retrospectively for visual comparison.
