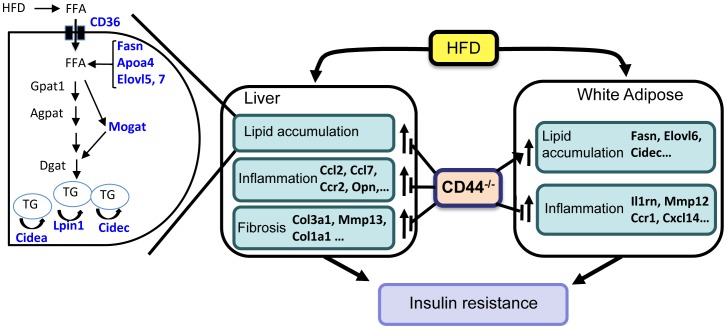
Figure 9. Schematic view of the links between CD44 deficiency and the development of diet-induced hepatic steatosis, inflammation, and fibrogenesis, adipose-associated inflammation, and insulin resistance.
In liver, HFD induces the expression of many lipogenic genes, including Cd36, Fasn, Elovl, Apoa4, Mogat and Cidec and Cidea, which enhance the transport, synthesis, and accumulation of triglycerides. In addition, the expression of several inflammatory genes, such as Ccl2, Ccl7, Ccr2, and Opn, and fibrotic genes, including Col3a1, Mmp13, and Col1a1, are elevated and related to the observed increase in hepatic inflammation and the onset of hepatic fibrogenesis. In adipose tissue, HFD induces expression of several lipogenic genes and inflammatory genes (e.g., Il1rn, Mmp12, Ccr1, Cxcl14). The induction of these genes is in part responsible for the increased lipid accumulation and inflammation in WAT. Hepatosteatosis and WAT-associated inflammation subsequently promote the development of insulin resistance and glucose intolerance. The reduced expression of lipogenic, inflammatory, and fibrogenic genes in CD44-deficient mice is linked to their resistance to develop diet-induced hepatosteatosis. The inhibition of inflammatory genes and of the infiltration of macrophages and CD8+ lymphocytes in WAT of CD44KO mice results in reduced WAT-associated inflammation, while elevated expression of lipogenic genes is at least in part related to the observed increase in adiposity. The inhibition of hepatosteatosis and WAT-associated inflammation protects CD44-deficient mice against the development of insulin-resistance and glucose intolerance.