Table 3. Kinetic parameters for URN1-FF at pH 5.7.
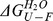 1(kcal mol−1)
1(kcal mol−1) |
mU-F 2(kcal mol−1M−1) | Cm 3 (M) | kF (s−1) | kU (s−1) | RTmU(kcal mol−1 M−1) | RTmF(kcal mol−1 M−1) | |
| 298 K | 5.57±0.28 | 0.78±0.04 | 7.13±0.55 | 2741±95 | 0.23±0.11 | 0.19±0.04 | 0.59±0.01 |
| 310 K | 5.28±0.04 | 0.81±0.04 | 6.50±0.34 | 7022±221 | 1.33±0.06 | 0.20±0.04 | 0.61±0.01 |
| FBP114 | 3.64±0.05 | 1.16±0.02 | 3.14±0.07 | 2200±90 | 3.66±0.06 | 0.154±0.003 | 1.01±0.02 |
Gibbs energy of unfolding with urea determined from the kinetic parameters.
Dependence of the Gibbs energy of unfolding with urea.
The urea concentration required to unfold 50% of the protein molecules.
The values of the FF domain of HYPA/FBP11 were previously reported at 283 K [19].
