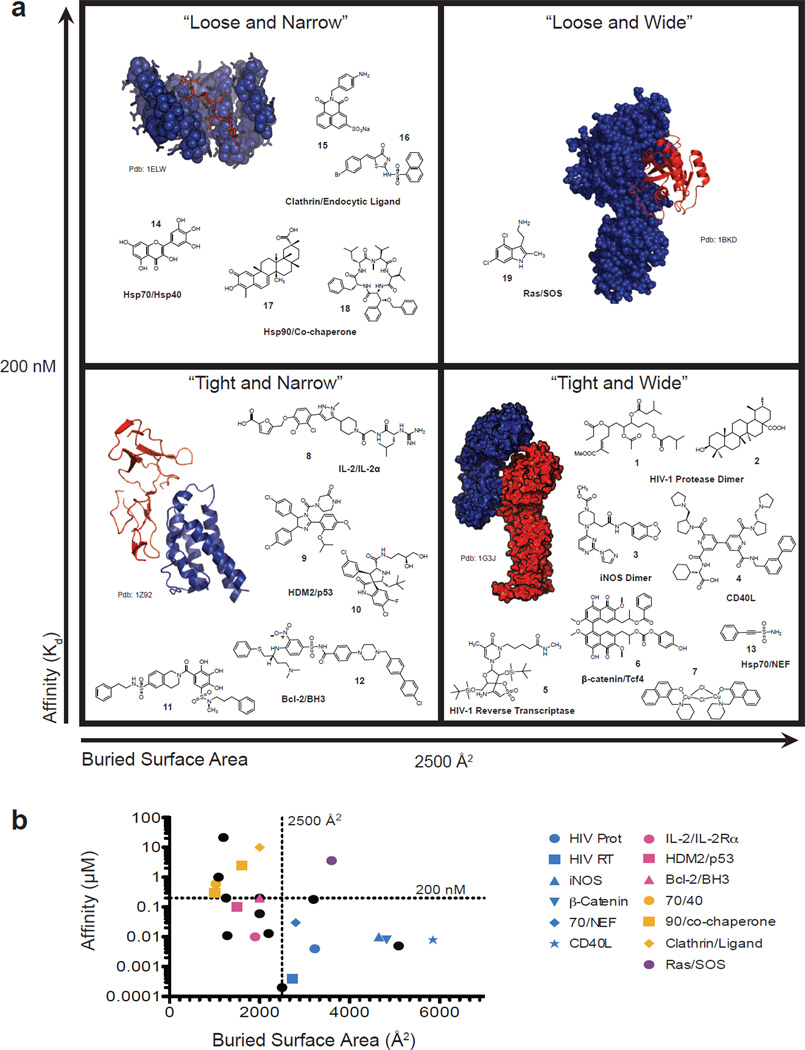
Figure 1. Categorization of protein-protein interactions (PPIs) and their inhibitors.
PPIs with known inhibitors were from obtained from the 2P2I (Ref. 10) and TIMBAL (Ref. 50) databases and recent literature. These PPIs were then categorized by the affinity (KD) of the protein-protein interaction and the buried surface area from co-crystal structures (see Table 1). These values were used to arbitrarily categorize the “inhibit-able” PPIs into four quadrants. To illustrate the types of proteins in each category, structures of representative PPIs from each class are shown, with each partner depicted in either blue or red. For “Tight and Wide” the interaction is between the armadillo repeat region of β-catenin and the catenin binding domain of Xenopus TCF3 (PDB: 1G3J). For “Tight and Narrow” the interaction is between IL-2 and the IL-2α Receptor (PDB: 1Z92). For “Loose and Narrow” the interaction is between TPR1 and a C-terminal peptide of Hsc70 (PDB: 1ELW) and for “Loose and Wide” the interaction is between Ras and SOS (PDB: 1BKD). Also shown are 19 representative chemical structures of the PPI inhibitors, illustrating the lack of consensus in molecular weight, shape or other characteristics.