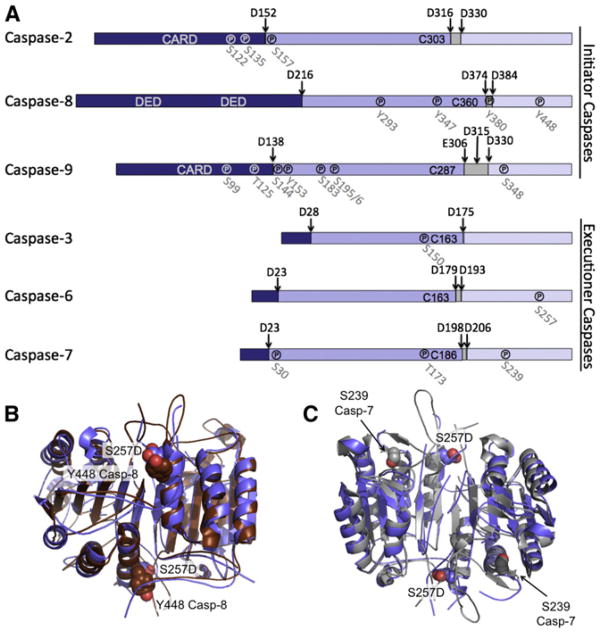
Figure 6. Phosphorylation Is Predicted to Misalign Active Site Loops in Many Caspases.
(A) Both initiator and executioner caspases are phosphorylated. Phosphorylation (℗) occurs at indicated sites in all domains of caspases: prodomain (dark blue), large subunit (medium blue), linker (gray), and small subunit (light blue). Interdomain cleavage sites are indicated by arrows. Phosphorylation typically results in inactivation.
(B) In caspase-8 Y448 phosphorylation leads to inactivation. Structural alignment of caspase-6 (blue) with caspase-8 (brown) shows capase-8 Y448 (brown spheres) is immediately adjacent to S257 in caspase-6 (blue spheres). Y448 phosphorylation is therefore predicted to lead to inhibition through a similar loop-misalignment mechanism.
(C) In caspase-7 phosphorylation of S239 results in inactivation. Structural alignment of caspase-6 (blue) with caspase-7 (gray) shows caspase-7 S239 (gray spheres) sitting at the base of the L3 loop. S239 phosphorylation is thus predicted to disrupt the folded state of L3 and prevent substrate binding.