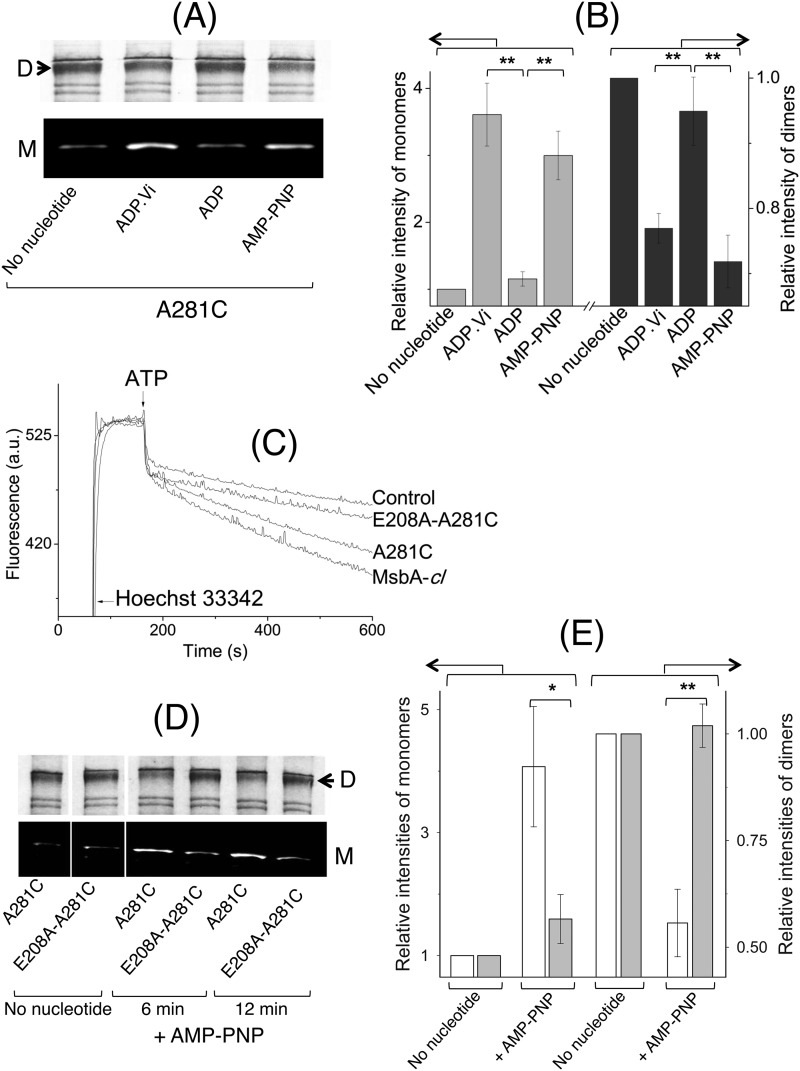
FIGURE 8.
Nucleotide-responsive cross-linking of A281C MsbA-cl and E208A/A281C MsbA-cl. A, intermolecular A281C-A281C′ cross-linking for A281C MsbA-cl was found to be nucleotide-responsive and thus could be used to monitor conformational changes associated with the ATPase cycle as a complement to previously published work with E208C MsbA-cl (10). All images here pertain to one and the same gel/experiment, processed in parallel to enhance readability (M, monomer; D, dimer). B, Atto590-labeled monomer and Coomassie Brilliant Blue-stained dimer intensities were analyzed by densitometry and are presented as -fold change in intensity relative to the no nucleotide condition (for which the -fold change was set at 1) (n = 3; **, p < 0.03, Student's t test). C, E208A mutation in the A281C background (E208A/A281C MsbA-cl) led to a reduction in Hoechst 33342 transport activity compared with the activity of MsbA-cl and A281C MsbA. A similar reduction was obtained for the E208A mutation in the WT MsbA background (see Fig. 3, A and B). D, compared with A281C MsbA-cl, the E208A/A281C MsbA-cl double mutant displayed a lack of changes in cross-linking Atto590 labeling in response to incubation with the nonhydrolysable ATP analog AMP-PNP (for 6 or 12 min). All panels pertain to one and the same gel/experiment, processed in parallel, and are cropped to enhance readability (M, monomer; D, dimer). E, E208A mutation interrupts the inward- to outward-facing conformational switch in MsbA. Densitometric analysis on the monomer and dimer signals for samples after 12 min of incubation without or with AMP-PNP was as indicated. Data are presented as -fold change in intensity, relative to the no nucleotide condition (for which the -fold change equals 1) (n = 3; *, p < 0.06; **, p < 0.04, Student's t test). Open bars refer A281C MsbA-cl, and gray bars refer to E208A/A281C MsbA-cl. Error bars represent mean ± S.E.