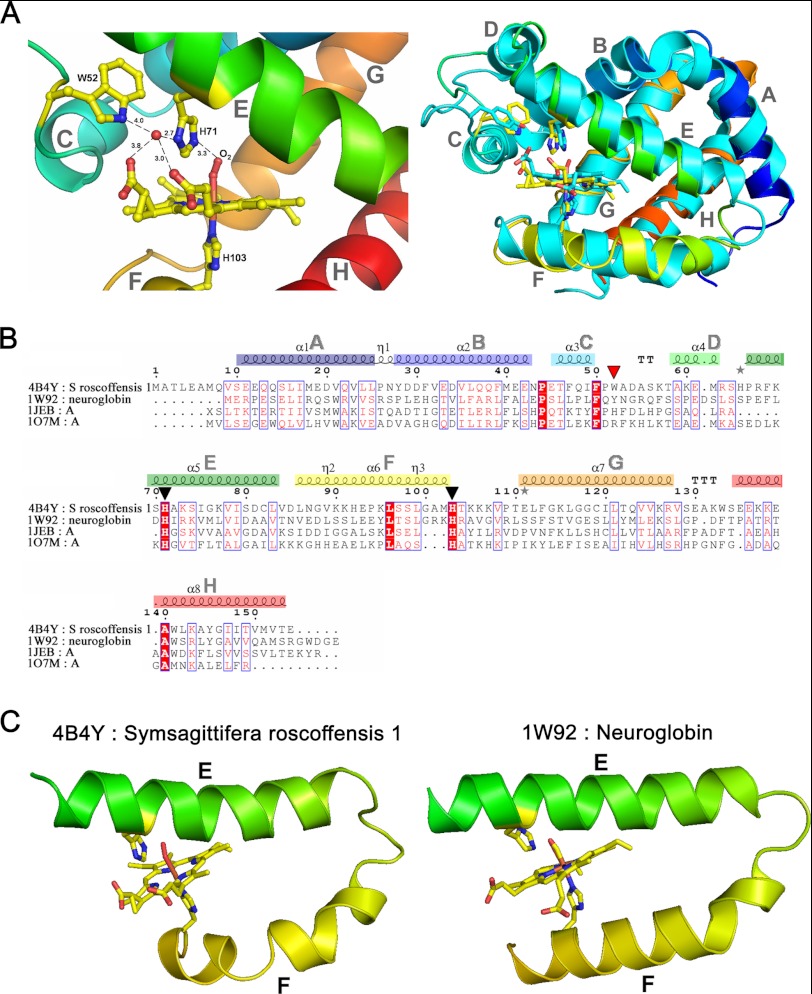
FIGURE 3.
A, ribbon representation of SrNgb1 crystal structure (4B4Y). Left, close-up view of the heme binding pocket of SrNgb1 highlighting the hydrogen bonding network involving the distal heme binding position and a tightly bound water molecule (HOH117). Right, three-dimensional structural super imposition with murine Ngb (1W92). B, multiple sequence alignment based on the structural superimposition with murine Ngb (1W92), bovine hemoglobin (1JEB), and sperm whale myoglobin (107M), as obtained with the program ESPRIPT. The conserved histidines (axial heme ligands) are marked by black triangles. The red triangle marks a Trp residue involved in the tight binding of a water molecule in the distal heme pocket. The eight helices that form the classical globin fold are labeled from A to H and color-coded from blue (N terminus) to red (C terminus) in the same manner as in the ribbon representation of SrNgb1 in Fig. 1B. C, extract of the SrNgb1 crystal structure highlighting the relative orientations of the heme-ligand containing helixes E and F. A proline at position 94 in helix F leads to a discontinuous and bent helix F in SrNgb1 (4B4Y). The same structural extract showing the relative orientations of helixes E and F in murine Ngb (1W92), where helix F is continuous and straight.