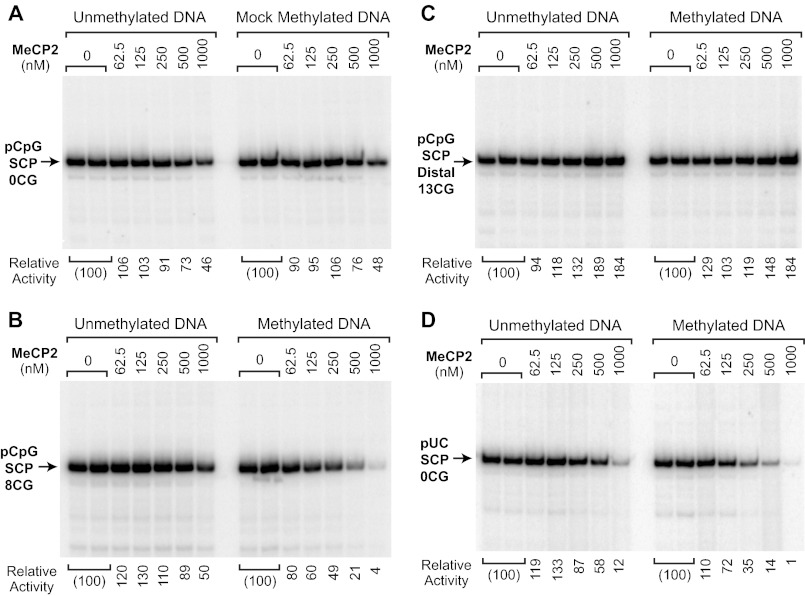
FIGURE 5.
CG methylation-specific repression by MeCP2 requires CG dinucleotides in the core promoter or the surrounding sequences. Transcription reactions were carried out with methylated and unmethylated template DNAs and the indicated concentrations of purified wild-type MeCP2. The DNA templates are depicted in Fig. 1B. Each image is a continuous single exposure of a series of reactions that were performed in parallel with equivalent amounts of methylated or unmethylated template DNA. At 500 nm MeCP2, there is approximately one molecule of MeCP2 per methyl-CG dinucleotide in the reaction mixture (see “Experimental Procedures”). The relative activity values are normalized to the amount of transcription observed in the absence of MeCP2. A, MeCP2 does not substantially repress transcription in the absence of CG dinucleotides. The pCpG-SCP-0CG plasmid lacks CG dinucleotides and, hence, cannot be methylated by M.SssI methyltransferase. In the mock methylation, the plasmid DNA was treated with M.SssI and S-adenosylmethionine under the standard methylation conditions. B, CG dinucleotides in the core promoter region are sufficient for transcriptional repression by MeCP2. The pCpG-SCP-8CG core promoter has eight CG dinucleotides in the core promoter region from −50 to +50 relative to the +1 transcription start site. C, MeCP2 does not repress transcription from a construct containing a stretch of 13 CG dinucleotides about 1.7 kbp upstream of a CG-deficient core promoter. D, a core promoter lacking CG dinucleotides can be repressed by MeCP2 via binding to the surrounding sequences. pUC-SCP-0CG lacks CG dinucleotides in the core promoter region (−50 to +50) but contains CG dinucleotides in the surrounding pUC vector sequences.