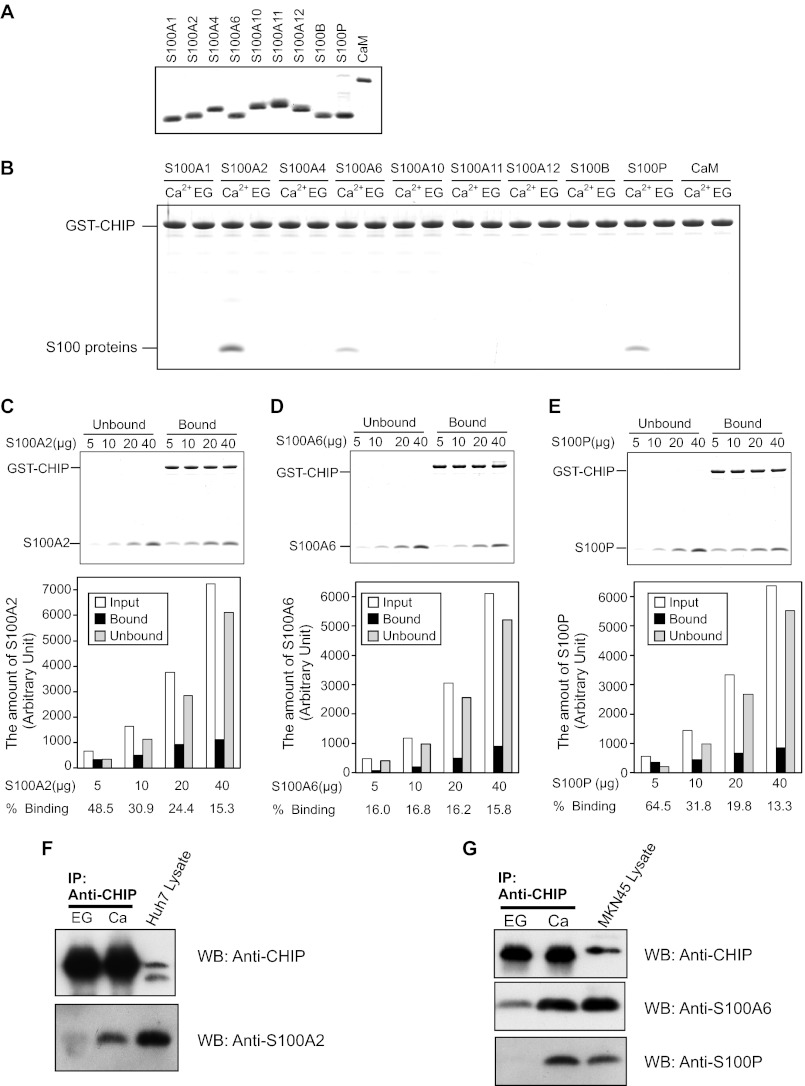
FIGURE 1.
Interaction of CHIP and S100 proteins. A, SDS-PAGE of S100 proteins and CaM used in the binding assays (2.5 μg/lane) are shown. B, GST pulldown assay was performed using wild-type GST-CHIP and the S100 proteins. The S100 proteins (20 μg) and GST-CHIP (20 μg) were incubated with glutathione-Sepharose 4B in Buffer A with 1 mm CaCl2 (Ca2+) or EGTA (EG). After agitation at 25 °C for 60 min, the beads were washed and then eluted with SDS-sample buffer. The eluted samples were analyzed by 10% Tricine-SDS-PAGE. The gel was stained with Coomassie Brilliant Blue. C–E, increasing amounts of S100A2 (C), S100A6 (D), and S100P (E) were mixed with GST-CHIP (20 μg), and pulldown assays were performed in the presence of 1 mm CaCl2. Unbound and bound proteins were analyzed by 10% Tricine-SDS-PAGE (upper panel). The gels were scanned, and arbitrary densitometric values (in pixels) were obtained. The amounts of S100 proteins (Input, Unbound, and Bound) are plotted. Percentages of bindings of the S100 proteins to GST-CHIP are listed below each bar (lower panel). F and G, Huh-7 lysates (F) and MKN-45 lysates (G) were immunoprecipitated with anti-CHIP antibody in the presence of 2 mm CaCl2 (Ca) or EGTA (EG). Immunoprecipitates were analyzed by Western blotting (WB) with the indicated antibodies.