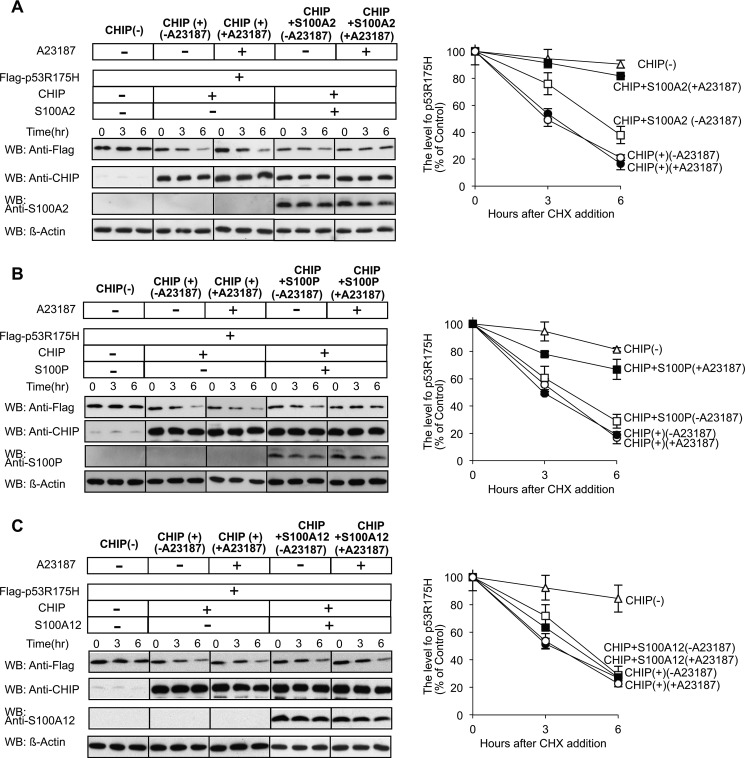
FIGURE 8.
Ca2+/S100 proteins suppress the degradation of mutant p53 by the CHIP-chaperone complex. A–C, left panel, Hep3B cells were transiently transfected with FLAG-p53R175H, CHIP, S100A2 (A), S100P (B), and S100A12 (C) as indicated. The transfected components of each dish are indicated on the top panels. The cells were treated with cycloheximide (CHX), with (+) or without (−) A23187. Lysates were prepared at time points 0, 3, and 6 h. The amount of p53R175H, CHIP, and S100s was analyzed by Western blotting (WB) with the indicated antibodies. Equal amounts of protein were loaded for each time point, and β-actin served as a loading control. A–C, right panel, the level of p53R175H was quantified. The relative amount of each protein present at t = 0 is expressed as 100% of the control. The error bars represent the S.E. with n = 3.