FIGURE 3.
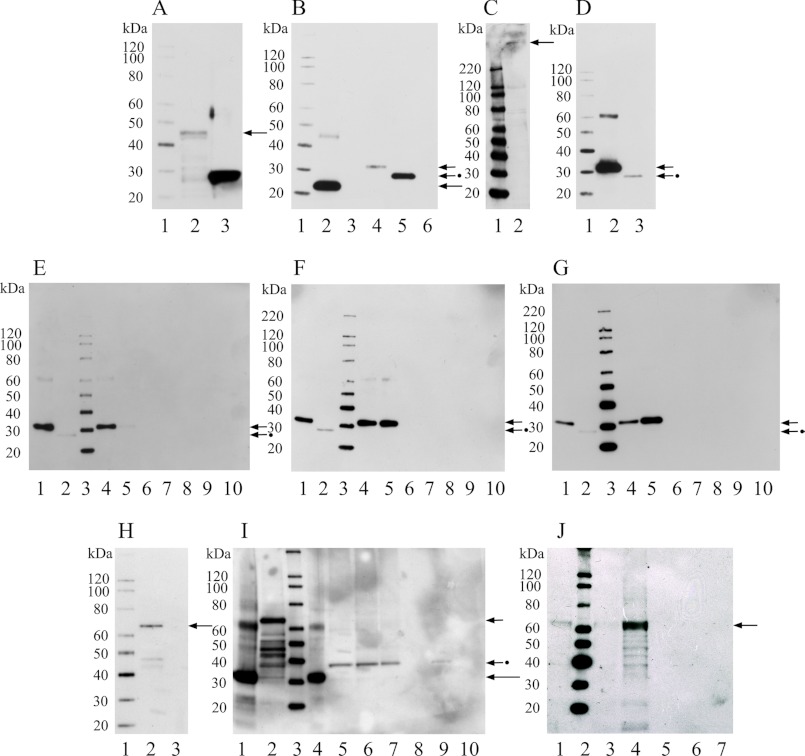
Western blot and protein interactions of rat CNGA3-N with CDH23 +68 and CDH23 −68 by GST pulldown assays. A, shown is a Western blot of fusion protein CNGA3-N in GST vector. Lane 1, Magic XP Western protein standards; lane 2, CNGA3 amino terminus in GST vector detected with anti-GST antibody (GE) at 42 kDa (arrow); lane 3, GST vector bacterial lysate (26 kDa), as negative control detected with anti-GST antibody (GE). B, shown is a Western blot of fusion proteins CNGA3-N in pRSET-B and rat CDH23 +68/−68 in pRSET-A, detected with anti-Xpress monoclonal antibody (Invitrogen). Lane 1, Magic XP Western standards; lane 2, affinity purified CNGA3-N at ∼20 kDa, long arrow; lane 3, pRSET-B bacterial lysate as negative control; lane 4, affinity-purified CDH23 +68 (aa 3134–3353, Fig. 2D) at 30 kDa, short arrow; lane 5, CDH23 −68 (aa 3134–3317, Fig. 2D) at 25 kDa, short arrow plus dot; lane 6, pRSET-A bacterial lysate as negative control. C, shown is a Western blot for full-length CDH23 from rat brain lysate detected with an affinity-purified goat polyclonal antibody raised against a peptide in the carboxyl terminus of CDH23 of human origin, crossing to rat sequence (sc-26338, Santa Cruz; 1:500). Lane 1, Magic XP Western standards; lane 2, full-length CDH23 at 365 kDa (arrow). D, shown is a Western blot for CDH23 +68 and CDH23 −68 in pRSET-A detected with anti-CDH23 antibody (sc-26338, Santa Cruz; 1:500). Lane 1, Magic XP Western standards; lane 2, affinity-purified CDH23 +68 at 30 kDa, short arrow; lane 3, RCDH23 −68 at 25 kDa, short arrow plus dot. E, shown is a Western blot for GST pulldown assay for GST-CNGA3-N with either CDH23 +68 or CDH23 −68 pRSET fusion proteins (200 and 50 ng) detected with anti-Xpress monoclonal antibody. Lane 1, CDH23 +68 protein (30 kDa), short arrow; lane 2, CDH23 −68 protein (25 kDa) used in the pulldown assay, short arrow plus dot; lane 3, Magic XP Western standards; lanes 4 and 5 indicate pulldown for CDH23 +68 (∼200 ng and ∼50 ng, respectively) with GST-CNGA3-N, short arrow; lanes 6 and 7 indicate no pulldown for CDH23 −68 (∼200 and ∼50 ng, respectively) with GST-CNGA3-N; lane 8, a negative control, indicates no pulldown for CDH23 +68 with GST-CNGA3-N but no beads; lanes 9 and 10 are additional negative controls (lane 9, GST lysate without CNGA3-N plus CDH23 +68 plus glutathione beads; lane 10, CDH23 +68 and glutathione beads), all detected with anti-Xpress monoclonal antibody. F, shown is a Western blot for a GST pulldown assay for GST-CNGA3-N with CDH23 +68 and −68 pRSET fusion proteins, similar to E except for the CDH23 protein concentrations used (100 and 150 ng, respectively). G, shown is a Western blot for a GST pulldown assay: GST-CNGA3-N with CDH23 +68 and CDH23 −68 fusion peptides detected with anti-CDH23 antibody (Santa Cruz, 1: 500). Lane 1, CDH23 +68, short arrow; lane 2, CDH23 −68 used in the pulldown assay, short arrow plus dot; lane 3, Magic XP Western standards; lanes 4 and 5 indicate pulldown for CDH23 +68 (∼50 and ∼200 ng, respectively) with GST-CNGA3-N, short arrow, whereas lanes 6 and 7 indicate no pulldown for CDH23 −68 (∼50 and ∼200 ng, respectively) with GST-CNGA3-N; lane 8 indicates no pulldown for CDH23 +68 with GST-CNGA3-N (no beads); lanes 9 and 10 are negative controls (lane 9, GST lysate without CNGA3 and CDH23 +68 and glutathione beads; lane 10, CDH23 +68 and glutathione beads). H, shown is a Western blot for myosin VIIa fusion protein. Lane 1, Magic XP Western standards; lane 2, myosin-VIIa at 70 kDa detected with anti-Xpress monoclonal antibody (Invitrogen), arrow; lane 3, pRSET-A bacterial lysate as negative control, detected with anti-Xpress monoclonal antibody (Invitrogen). I, shown is a Western blot for competitive GST pulldown assay between CDH23 +68 and myosin VIIa fusion proteins in binding to GST-CNGA3-N. Lane 1, CDH23 +68 fusion protein at 30 kDa, long arrow; lane 2, myosin VIIa lysate used in the pulldown assay, myosin VIIa at 70 kDa (short arrow); lane 3, Magic XP Western standards; lane 4, pulldown of CDH23 +68 at 30 kDa (∼300 ng) by GST-CNGA3-N in the absence of myosin VII (∼100 ng, long arrow) lysate; lanes 5 and 6 (duplicates) indicate that in the presence of myosin VIIa (∼100 ng), CDH23 +68 (∼100 ng) at 30 kDa is not pulled down. However, a protein with a higher molecular mass of ∼38 kDa appears, putatively associated with myosin VIIa lysate (compare lane 2 with lanes 5 and 6, short arrow + dot); lane 7, myosin VIIa lysate protein in pRSET vector and GST-CNGA3-N. A protein with higher molecular mass potentially deriving from myosin VIIa (∼100 ng) lysate, compare lanes 2 and 7, interacts with GST-CNGA3-N fusion protein on glutathione beads. Lane 8, negative control consisting of GST-CNGA3-N with CDH23 +68 and myosin VIIa, where no beads were added; lanes 9 and 10 are negative controls: lane 9 indicates results for GST lysate (no CNGA3), glutathione beads, myosin VIIa and CDH23 +68; lane 10 illustrates results for glutathione beads, myosin VIIa, and CDH23 +68. J, shown is a Western blot for pulldown assay of GST-CNGA3-N with myosin VIIa pRSET fusion protein detected with anti-Xpress antibody. Lane 1, myosin VIIa standard (70 kDa), arrow; lane 2, Magic XP western standards; lanes 3 and 4 indicate pulldown for myosin VIIa (∼50 and ∼150 ng, respectively, arrow) with GST-CNGA3-N, arrow; lane 5 indicates no pulldown for myosin VIIa (∼150 ng) with GST-CNGA3-N (no beads); lanes 6 and 7 are negative controls. Lane 6 indicates results for GST lysate (no CNGA3) plus glutathione beads plus myosin VIIa at ∼150 ng; lane 7 shows results for glutathione beads plus myosin VIIa at ∼ 150 ng.