FIGURE 2.
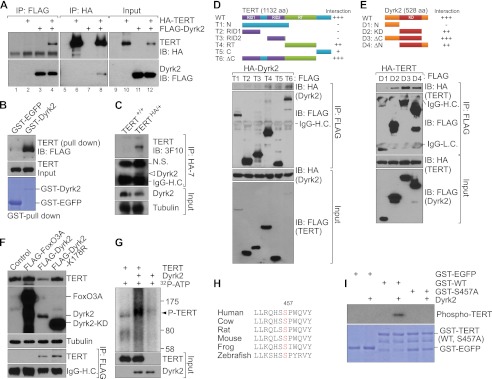
Dyrk2 binds to TERT and phosphorylates its serine residue. A, binding of Dyrk2 to TERT. 293T cells were transiently transfected with FLAG-TERT and HA-Dyrk2 plasmids. Twenty-four hours later, 293T cell lysates were analyzed using reciprocal IP with either anti-HA or anti-FLAG antibodies. Dyrk2-TERT interaction was detected (lane 4, upper panel; lane 8, lower panel). B, direct interaction of Dyrk2 with TERT. Purified GST-EGFP (control) and GST-Dyrk2 proteins were used for a GST pull-down assay by incubating each protein with HeLa-TERT cell lysates. The resulting GST-protein complex was resolved using IB (FLAG). C, association of endogenous TERT with Dyrk2. Endogenous TERT-Dyrk2 interaction was analyzed using IP (HA) and IB (Dyrk2) with parental (TERT+/+) and TERT knockin (TERTHA/+) mouse embryonic stem cells. D and E, binding domain mapping of the TERT-Dyrk2 interaction. D, various FLAG-TERT fragments and full-length HA-Dyrk2 plasmids were cotransfected into 293T cells. After 36 h, each lysate of these cells was analyzed using IP (FLAG) and IB (HA). Of note is that T4, T5, and T6 deletion mutants interacted with Dyrk2, indicating that a C-terminal domain including the RT domain is necessary for TERT-Dyrk2 interaction. RID1 and 2, RNA-interacting domain 1 and 2; N, N terminus; C, C terminus; ΔC, C terminus-deleted mutant. E, each FLAG-Dyrk2 fragment with full-length HA-tagged TERT (HA-TERT) was cotransfected into 293T cells. Lysates of these cells were analyzed using IP (FLAG) and IB (HA). D1 fragment lacking a kinase domain failed to interact with TERT protein. Also, deletion of the C terminus in Dyrk2 was dispensable for TERT interaction, indicating that the Dyrk2 kinase domain is sufficient for TERT interaction. N, N terminus; KD, kinase-dead; ΔC, ΔC terminus-deleted mutant; ΔN, N terminus-deleted mutant. F, kinase activity of Dyrk2 is not required for TERT-Dyrk2 interaction but is necessary for TERT protein degradation. In HeLa cells stably expressing HA-TERT, the plasmids FOXO3A, Dyrk2, and Dyrk2-kinase dead mutant were transiently transfected (24 h) for assessment of TERT protein expression using IB (HA) and TERT association with Dyrk2 (IP, FLAG; IB, HA). Of note, whereas the KD mutant still bound to TERT (lanes 4, lower panel), it did not down-regulate TERT protein expression (lane 4, top panel). G, Dyrk2 phosphorylation of TERT protein. In vitro transcribed and translated TERT (substrate) and Dyrk2 (kinase) were incubated with [32P]ATP. Dyrk2 phosphorylated TERT (lane 2, arrowhead). H, evolutionary conservation of the Dyrk2 consensus substrate sequence (R/KXX(X)S/TP) in TERT in vertebrates. Serine (red): candidate phosphorylation site for Dyrk2. I, phosphorylation of TERT at Ser457 by Dyrk2. GST-EGFP (control), wild-type TERT, and S457A (mutant) TERT fragments were incubated with Dyrk2 for in vitro kinase assay. GST-EGFP served as a negative control for the kinase reaction. Wild-type TERT was phosphorylated by Dyrk2 (lane 4), but the S457A mutant was not (lane 6).
