FIGURE 3.
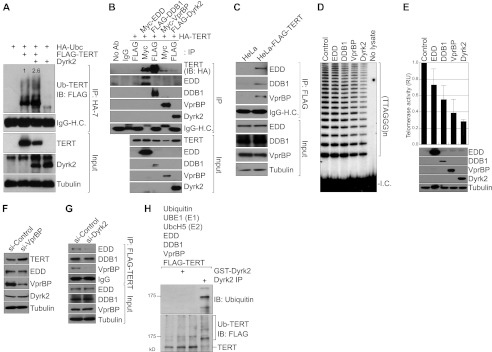
The Dyrk2-EDVP E3 ligase complex ubiquitinates TERT for degradation. A, Dyrk2-increased TERT ubiquitination. HeLa cells were transiently transfected with UBC, TERT, and Dyrk2 plasmids. After 36 h, cells were collected for IP (HA-tagged UBC) and IB (FLAG-TERT) analysis. Dyrk2 down-regulated the total level of TERT protein expression (lanes 2 and 3, input), and increased TERT ubiquitination (lanes 2 and 3, IP). Blots were normalized by TERT input and quantified using ImageJ. B, association of the EDVP E3 ligase complex with TERT. 293T cells transfected with HA-TERT, Myc-tagged EDD, FLAG-tagged DDB1, Myc-tagged VprBP, or FLAG-tagged Dyrk2 were analyzed using IP (FLAG or Myc) and IB (HA). Of note is that each component of the EDVP complex bound to TERT protein (lanes 4–7, top panel). C, association of TERT with endogenous EDVP E3 ligase components. HeLa-TERT cell lysates were analyzed using IP and IB. D and E, inhibition of telomerase activity by the EDVP E3 ligase complex. 293T cells transiently transfected with each EDVP component were analyzed for telomerase activity using TRAP assays (D). To quantify the effects of Dyrk2-EDVP on telomerase activity, the TRAP assay results were quantified using the ImageJ software program (E) and plotted (n = 3). The error bars indicate S.D. RU, relative unit. F, VprBP depletion stabilizes TERT protein. HeLa-TERT cells transfected with VprBP siRNA were analyzed using IB. Of note is that knockdown of VprBP expression increased the level of TERT protein, indicating an endogenous function of VprBP in TERT destabilization. G, mediation of the TERT-EDVP E3 ligase complex by Dyrk2. HeLa-TERT cells were transfected with a control siRNA (si-Control) or Dyrk2 siRNA (si-Dyrk2), and analyzed using IP (FLAG) and IB. Under Dyrk2-depleted conditions, both EDD and VprBP exhibited decreased binding to TERT. Of note is that DDB1-TERT interaction was not affected by Dyrk2 shRNA, indicating an EDVP-independent DDB1 association with TERT. H, requirement of Dyrk2 for EDVP E3 ligase-mediated TERT ubiquitination. For in vitro ubiquitination assays of TERT, E1, E2, and E3 components, and Dyrk2 were incubated with TERT protein. Next, ubiquitinated TERT was analyzed using IB (FLAG and ubiquitin). In the presence of Dyrk2, TERT was ubiquitinated (lane 4). Of note, bacterially expressed GST-Dyrk2 failed to ubiquitinate TERT because of a lack of post-translational modification of GST-Dyrk2 activity (tyrosine phosphorylation in the activation loop of Dyrk2; lane 2).
