FIGURE 4.
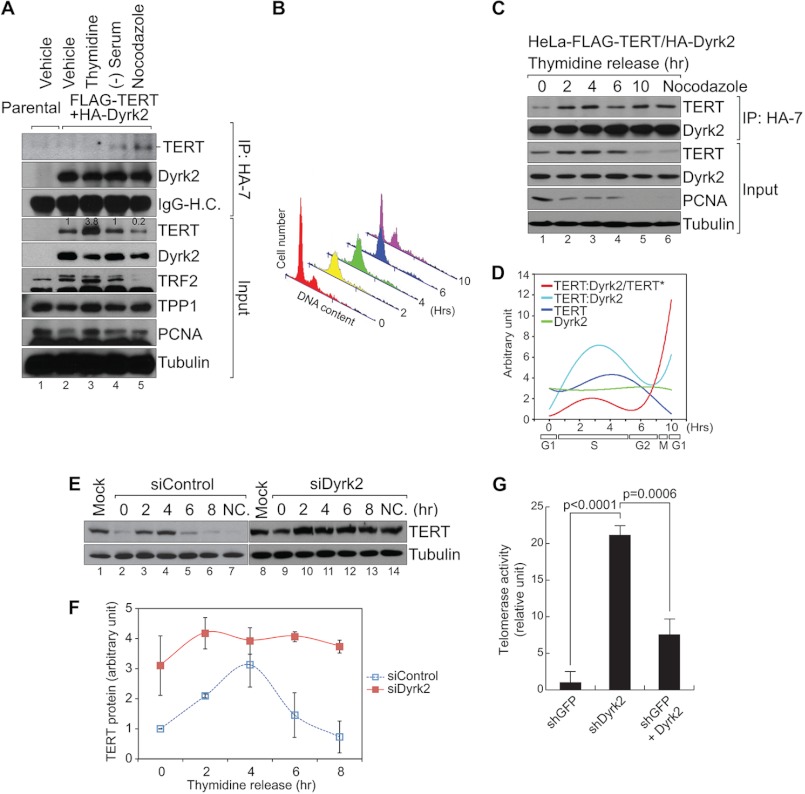
Cell cycle-dependent TERT regulation by Dyrk2. A, cell cycle of HeLa (GFP-expressing control cells) and HeLa-FLAG-TERT + HA-Dyrk2 cells was arrested by treatment with thymidine (2 mm, 24 h), serum starvation (48 h), or nocodazole (100 μm, 14 h). Cell lysates from each condition were analyzed by co-immunoprecipitation (HA) and immunoblotting (FLAG) assays. TERT protein level was inversely correlated with the TERT-Dyrk2 association (see lanes 3 and 5). Blots were quantified using ImageJ. B—D, HeLa-FLAG-TERT + HA-Dyrk2 cells were synchronized using thymidine double block (200 nm, more than 17 h each) and released for cell cycle progression. At each time point (0, 2, 4, 6, and 10 h), cells were analyzed using flow cytometry (B) and co-immunoprecipitation (HA)-immunoblotting (FLAG) assays (C), physical association between TERT and Dyrk2 during the cell cycle was quantified and plotted (ImageJ) (D) (n = 2). When the TERT protein level decreased during the G2/M phase, the TERT-Dyrk2 association increased. TERT:Dyrk2/TERT indicates the normalized TERT-Dyrk2 interaction by total TERT protein level (asterisk). E and F, HeLa-TERT cells transiently transfected with siControl or siDyrk2 were synchronized by thymidine double block and released for cell cycle progression. At different time points (0, 2, 4, 6, and 8 h), HeLa-TERT cells were analyzed using immunoblotting assays for TERT protein (E). TERT protein levels were quantified using ImageJ and plotted (n = 3) (F). Compared with the control (lanes 2–8), Dyrk2-depleted HeLa-TERT cells exhibited an overall increase in TERT protein levels (lanes 10–14). Error bars, S.D.; NC: nocodazole treatment (100 μm, 14 h). G, hyperactivation of endogenous telomerase activity by Dyrk2 knockdown. MCF-7 cells were stably transduced with lentivirus expressing shGFP (control) or shDyrk2. shDyrk2 non-targetable Dyrk2 retrovirus was also stably transduced for reconstitution assay (lane 3). Cell lysates were prepared for TRAP using real-time PCR. n = 3; Student's t test; error bars, S.D.