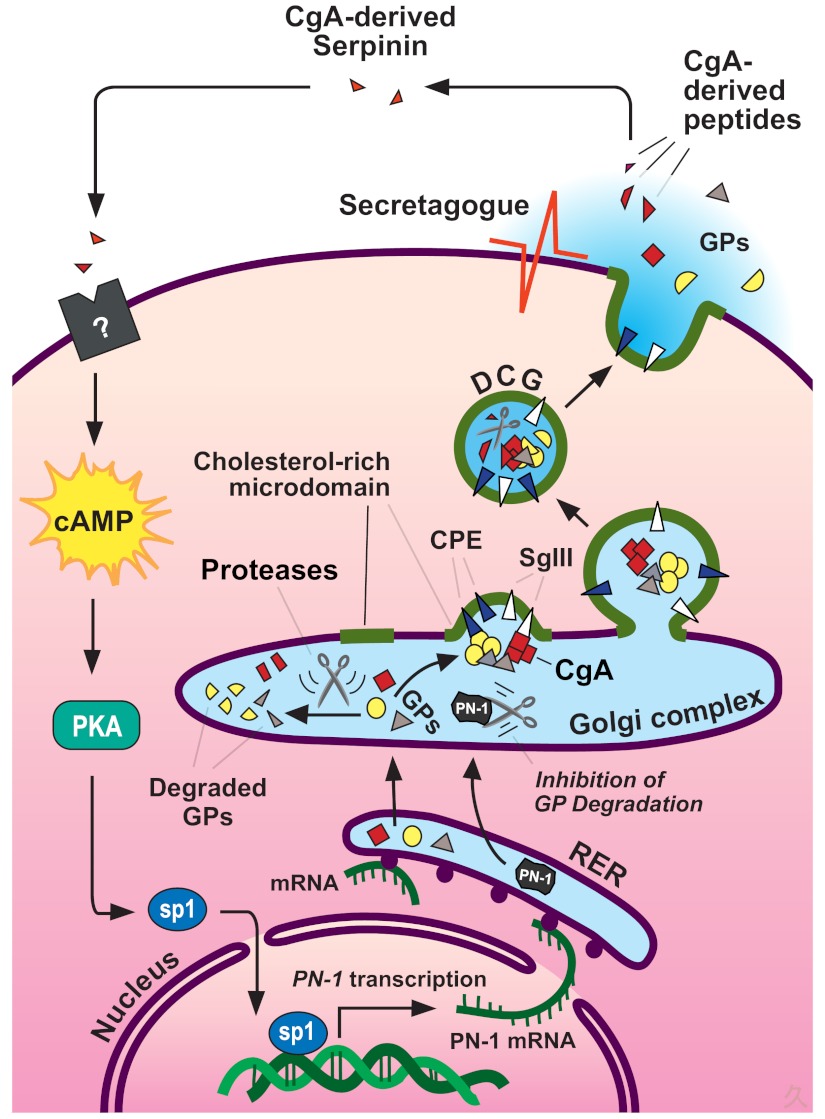
Fig. 5.
Model for intracellular trafficking of granule proteins and autocrine regulation of DCG biogenesis by the CgA-derived peptide serpinin in endocrine cells. Granule proteins (GPs, including granins and prohormones) are synthesized at the RER and then transported to the Golgi complex where they are sorted at the TGN into the regulated secretory pathway. Granins (e.g., CgA) and prohormones form aggregates, which bind to SgIII or carboxypeptidase E (CPE), sorting receptors that are anchored to cholesterol-sphingolipid-rich membrane microdomains at the TGN. These membrane domains bud under the driving force of the granin (CgA and CgB) aggregates to form immature granules. Specific proteolytic enzymes process the prohormones fully or the granins partially to yield biologically active peptides. The granins, the major protein in the granules, condense to form mature DCG. Excess granule proteins are degraded in the Golgi complex. Upon stimulation of the cell, DCG exocytose and release their contents. In cells expressing CgA, a C-terminal peptide, serpinin, is released and binds to a putative G protein-coupled receptor to increase the transcription and biosynthesis of protease nexin-1 (PN-1) via a cAMP-PKA-Sp1 signal transduction pathway. PN-1 inhibits GP degradation to increase GP levels, which in turn leads to more DCG formation to replenish the ones secreted.